Abstract
We investigated whether nilvadipine has a neuroprotective effect on retinal ganglion cells (RGCs) in a mouse model of ocular hypertension (OH) that expresses cyan fluorescein protein (CFP) in RGCs. OH was induced in the right eyes of Thy1-CFP transgenic mice using a laser. Nilvadipine or vehicle treatment began simultaneously with OH modeling and was administered intraperitoneally once daily for 8 weeks. Intraocular pressure (IOP) in both the laser- and non-treated eyes was measured weekly with the microneedle method, and calculations were performed to estimate the pressure insult in each eye. Using a retinal whole mount, the number of RGCs was counted at week 9. Laser-treated eyes showed a significant increase in IOP (p < 0.01), and the pressure insult did not differ between the drug-treated groups. Over time, laser treatment produced a significant decrease in the number of RGCs in the vehicle-treated groups, but this effect was attenuated by nilvadipine treatment. The pressure insult and RGC survival rate were significantly negatively correlated in the vehicle-treated group (y = -0.078 x + 107.8, r = 0.76, p < 0.001), but not in the nilvadipine-treated group (y = -0.015 x + 99.9, r = 0.43, p = 0.128). Nilvadipine was a potent neuroprotective agent for RGCs in our mouse model of OH and may have potential for protection against glaucoma. This model is useful as a screening tool for drugs with retinal protective effects.
Keywords: Glaucoma, Neuroprotection, Nilvadipine, Calcium channel blocker, Mouse
1. Introduction
There are approximately 60 million glaucoma patients worldwide, of whom 8.4 million suffer from bilateral blindness [1,2]. Glaucoma is the leading cause of blindness worldwide, and primary open-angle glaucoma is estimated to affect approximately 3% of the population aged 40–80 years (44.1 million people) [3]. While multiple factors may be involved in the onset of glaucomatous optic neuropathy (GON), intraocular pressure (IOP) reduction is currently the only effective treatment [[3], [4], [5]]. GON often progresses despite this treatment, and neuroprotective therapy is a promising new alternative for reducing IOP. However, unlike neurodegenerative disorders, such as cerebral infarction [6], Parkinson’s disease [7], and Alzheimer’s disease [8,9], neuroprotective therapies for glaucoma are unproven [10].
Calcium channel blockers (CCBs) have been approved for the treatment of hypertension and brain dysfunction. They increase vasodilation, decrease vascular resistance, and ultimately decrease blood pressure and increase blood flow by inhibiting calcium influx into smooth muscle cells in blood vessel walls. Studies have shown the potential of CCBs for the treatment of glaucoma. In patients with normal tension glaucoma (NTG), nifedipine [11], brovincamine [12], diltiazem, nifedipine, verapamil [13], and nilvadipine [14] inhibited visual field progression. In addition, nifedipine and brovincamine increased peripheral blood flow in the optic disc [11,12], diltiazem, nifedipine, and verapamil ameliorated optic nerve damage [13], and nilvadipine increased posterior choroidal circulation over 3 years [14]. These data indicate that CCBs may inhibit the progression of glaucoma by improving blood flow. Among these CCBs, nilvadipine exhibits superior drug retention, and has the most lipophilic structure [[15], [16], [17]] and highest retinal accumulation rate [18]. For these reasons, although its neuroprotective effects are not fully understood, nilvadipine is a promising treatment for glaucoma.
We evaluated the neuroprotective potential of nilvadipine treatment in a mouse model of chronic ocular hypertension (OH). We previously established a laser-treated OH mouse model, and assessed pressure-dependent retinal ganglion cell (RGC) damage using transgenic mice expressing cyan fluorescein protein (CFP) in RGCs [19,20] In our model, RGC death can be assessed by fluorescence microscopy without invasive injection of fluorescent dyes from the superior colliculus.
2. Materials and methods
2.1. Animals
This study was approved by the Research Ethics Committee of the Graduate School of Medicine and Faculty of Medicine at the University of Tokyo, and animals were treated and studied according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Animal studies are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). B6. Cg-TgN (Thy1-CFP) 23 Jrs/J mice carrying the Thy1 promoter linked to a CFP reporter were obtained from the Jackson Laboratory (Bar Harbor, MN, USA). Mice were bred and housed in clear cages loosely covered with air filters. The cages contained white bedding. The environment was maintained at 21 °C with a 12 h:12 h light-dark cycle. All mice were fed ad libitum. All experiments were conducted in male mice aged ≥18 weeks.
2.2. Induction of experimental ocular hypertension
The aqueous outflow from the right eye of each animal was blocked by laser photocoagulation of the limbus, as described previously [19,20]. In summary, the right eye was subjected to laser-induced OH (laser-treated eye), and the left eye (non-treated eye) was used as a control. After anesthesia via an intraperitoneal injection of a solution containing ketamine (100 mg/kg) and xylazine (9 mg/kg), the pupil was dilated by instillation of 5 μL of 0.5% tropicamide and 0.5% phenylephrine hydrochloride (Mydrin-P; Santen Pharmaceutical, Osaka, Japan). Next, the anterior chamber was flattened via aspiration of aqueous humor, and laser photocoagulation (wavelength: 532 nm, output: 200 mW, duration: 0.05 s, spot size: 200 μm) was performed around the entire limbus. After the procedure, antibiotic ointment was applied topically to prevent infection. Additionally, topical corticosteroid eye drops (betamethasone 0.1%, Rinderon; Shionogi Inc., Osaka, Japan) were applied for 7 days to prevent inflammation. The IOP measured 1 week after laser treatment was used to determine the success of the laser-induced ocular hypertension model. In this model, IOP fluctuates widely and may show normal IOP in some cases, but still increases in the following weeks. Therefore, laser-treated eyes with normal IOP were also used. Mice were not used if the IOP increased >40 mmHg or decreased to <10 mmHg at the first IOP measurement.
2.3. Drug administration
Several drugs were administered simultaneously with the induction of OH. Nilvadipine (Wako, Osaka, Japan) was dissolved in a mixture of ethanol, polyethylene glycol 400, and distilled water (2:1:7) at a concentration of 0.1 mg/mL, along with physiological saline, before use [21]. After weighing, nilvadipine was administered intraperitoneally (0.1 mg/kg) to 30 mice (nilvadipine group), once daily for 8 weeks. Vehicle without nilvadipine was administered in the same manner (vehicle group).
2.4. IOP measurement
The IOP was measured weekly using a microneedle method during drug administration, as described previously [19]. Briefly, a borosilicate glass microneedle (tip diameter: 75–100 μm, outer diameter: 1.0 mm; World Precision Instruments [WPI], Sarasota, FL, USA) was assembled into a pressure transducer (model BLPR; WPI). The pressure detected by the transducer was recorded by the data acquisition and analysis system (PowerLab; ADInstruments, Colorado Springs, CO, USA). A microneedle was placed in the anterior chamber of each eye and pressure was recorded after anesthesia.
2.5. Calculation of pressure insult
For each animal, the change in IOP over time was plotted for both laser- and non-treated eyes, and the area under each curve was calculated in units of mmHg × day. The magnitude of IOP elevation was calculated as the difference between these regions. The pressure insult caused by an elevated IOP was defined as the difference in IOP between the laser-treated eye higher and non-treated eye over the study period [20].
2.6. RGC count
Mice were sacrificed 9 weeks after laser photocoagulation. The eyes were enucleated and immediately fixed for 1 h at 4 °C in 0.1 M phosphate-buffered saline containing 4% paraformaldehyde. Four radial incisions were made to flatten the retina for mounting on a glass slide with coverslips, and images were acquired using a fluorescence microscope (BX 50; OLYMPUS, Tokyo, Japan) with a CFP filter set. The number of CFP-expressing RGCs was counted manually in 12 separate regions at 40,000 μm2 using image processing software (ImageJ; National Institutes of Health, Bethesda, MD, USA) as described previously [19]. In detail, samples were taken from the optic disc in each retinal quadrant at 600 (central), 1200 (central), and 1800 (peripheral) μm, and the mean density of RGCs (RGCs/mm2) was calculated for each area. RGC density was statistically compared between laser- and non-treated eyes for each drug treatment group. The RGC survival rate in laser-treated eyes was defined according to the normalized RGC density for non-treated eyes. The correlation between pressure insult and RGC survival rate was evaluated by regression analysis.
2.7. Statistical analysis
Data are expressed as mean ± standard deviation. Paired Student’s t-test was used to compare IOP in the laser- and non-treated eyes. Unpaired Student’s t-test was used to compare body weight of mice in groups. Paired Student’s t-test and unpaired Student’s t-test were used to compare area under each curve value of the laser- and non-treated eyes in groups calculated in units of mmHg-days. Paired Student’s t-test and Aspin-Welch test were used to compared RGC numbers. A linear mixed model was used to compare the correlation coefficients between pressure insult and RGC survival rate in each retinal area; p < 0.05 was considered statistically significant.
3. Results
3.1. Number and body weight of mice
IOP measured 1 week after laser treatment was used to determine the success of the laser-induced ocular hypertension model. Some eyes were excluded because they developed inflammatory ocular atrophy after laser treatment (nilvadipine group: n = 9, vehicle group: n = 5) or had elevated IOP >40 mmHg that was too high to use as a glaucoma model (nilvadipine group: n = 7, vehicle group: n = 2). OH was successfully induced in 23 of 30 mice in the vehicle group and 14 of 30 mice in the nilvadipine group. Data from ocular hypertensive mice were analyzed. The body weight ranges of the vehicle (25.4–27.5 g) and nilvadipine (24.8–26.8 g) groups were not significantly different throughout the 8-week treatment period (unpaired Student’s t-test) (Fig. 1).
Fig. 1.
Changes of mouse body weight. Data are mean ± SD. There was no significant difference between vehicle and nilvadipine-treated mice during the study period.
3.2. Induction of experimental OH
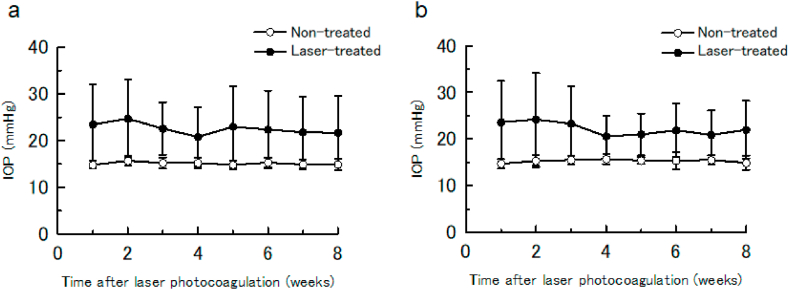
In the vehicle group, the mean IOP (23.5 ± 8.6 mmHg) in laser-treated eyes was significantly higher than in non-treated eyes (14.8 ± 0.8 mmHg) at 1 week, and remained significantly higher at 8 weeks after laser treatment (paired Student’s t-test; p < 0.001) (Fig. 2(a) and Supplementary Table S1). In addition, the mean IOP in both groups remained stable throughout the 8-week study period (laser therapy: 20.8–24.7 mmHg; non-treated: 14.8–15.7 mmHg). Similarly, in the nilvadipine group, the mean IOP (22.0 ± 6.2 mmHg) in laser-treated eyes was significantly higher than that in non-treated eyes (14.9 ± 1.5 mmHg) at 1 week, and remained significantly higher at 8 weeks after laser treatment (paired Student’s t-test; p < 0.01) (Fig. 2(b) and Supplementary Table S1). Again, the mean IOP in both groups remained stable throughout the 8-week study (laser treatment: 20.6–24.2 mmHg; non-treated: 14.7–15.7 mmHg).
Fig. 2.
Time course of average intraocular pressure (IOP) in the laser- and non-treated eyes. Data are mean ± SD. Vehicle group (a). Nilvadipine group (b). Laser-treated eyes had significantly higher IOP than non-treated eyes in both the vehicle and nilvadipine groups.
3.3. Pressure insult of laser- and non-treated eyes
The area under each curve value calculated for the laser-treated eyes were significantly higher than those of the non-treated eyes in both drug-treated groups (vehicle non-treated: 730 ± 31, vehicle laser-treated: 1085 ± 256, paired Student’s t-test; p < 0.001; nilvadipine non-treated: 739 ± 37, nilvadipine laser-treated: 1059 ± 253, paired Student’s t-test; p < 0.001) (Table 1 and Supplementary Table S2). There was no significant difference in the level of area under each curve value between vehicle group and nilvadipine group when in either the non-treated or laser-treated eyes (unpaired Student’s t-test; vehicle: p = 0.414; nilvadipine: p = 0.761).
Table 1.
Area under each curve (AUC) of the laser- and non-treated eyes calculated in units of mmHg-days.
| Non-treated |
Laser-treated |
|||
|---|---|---|---|---|
| Vehicle | Nilvadipine | Vehicle | Nilvadipine | |
| AUC | 730 ± 31 | 739 ± 37 | 1085 ± 256*** | 1059 ± 253*** |
***p < 0.001 compared with non-treated AUC by paired Student’s t-test.
3.4. RGC densities
RGC densities were calculated for the central (600 μm), middle (1200 μm), peripheral (1800 μm), and whole areas of the retina. In the vehicle group, all four RGC densities were significantly lower in the laser-treated than non-treated eyes (paired Student’s t-test; p < 0.001 for all areas) (Table 2 and Supplementary Table S2). In contrast, in the nilvadipine group, no area showed significantly different RGC densities between the laser and non-treated eyes (paired Student’s t-test; p = 0.094, 0.150, 0.171, and 0.057, respectively). RGC densities in non-treated eyes were not significantly different between the groups (Aspin-Welch test; p = 0.716, 0.937, 0.100, and 0.507, respectively), but this was not the case for laser-treated eyes. In fact, the overall RGC densities were significantly higher in the nilvadipine than vehicle group (Aspin-Welch test; p = 0.020, 0.014, 0.021, and 0.014, respectively).
Table 2.
Retinal ganglion cell (RGC) numbers of central, middle, and peripheral areas, apart from the optic disc and whole area of the retina.
| RGC numbers (RGCs/mm2) |
|||||
|---|---|---|---|---|---|
| Non-treated |
Laser-treated |
||||
| Vehicle | Nilvadipine | Vehicle | Nilvadipine | ||
| Central | 1449 ± 150 | 1466 ± 970 | 1139 ± 376*** | 1361 ± 176§ | |
| Middle | 1471 ± 161 | 1467 ± 120 | 1181 ± 366*** | 1401 ± 139§ | |
| Peripheral | 1465 ± 137 | 1389 ± 127 | 1148 ± 326*** | 1336 ± 140§ | |
| Whole | 1462 ± 120 | 1440 ± 690 | 1156 ± 351*** | 1366 ± 131§ | |
***p < 0.001 compared with the RGC numbers of the non-treated eyes in vehicle group by paired Student’s t-test. §p < 0.05 compared with the RGC numbers of the laser-treated eyes in vehicle group by Aspin-Welch test.
3.5. Correlation between pressure insult and RGC survival rate
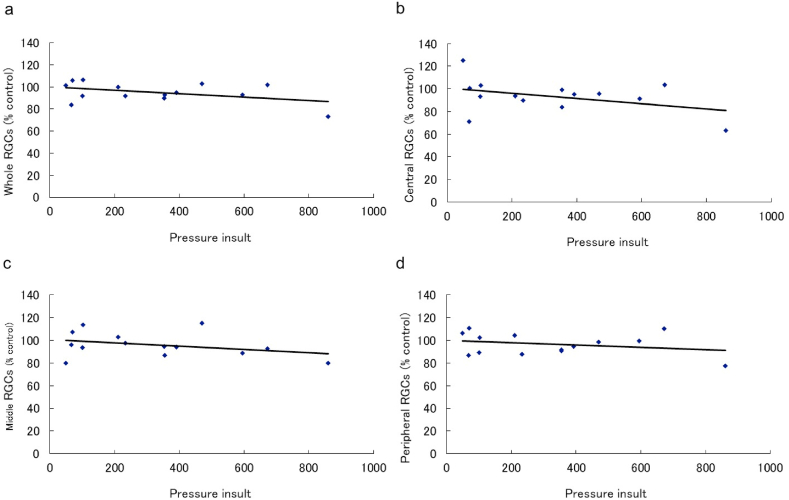
Regression analysis showed a significant negative correlation between pressure insult and RGC survival rate in all measurement areas in the vehicle group (whole area: y = -0.078 x + 107.8, r = 0.76, p < 0.001; central (600): y = -0.084 x + 109.9, r = 0.71, p < 0.001; middle (1200): y = -0.080 x + 109.6, r = 0.78, p < 0.001; peripheral: y = -0.071 x + 105.0, r = 0.71, p < 0.001) (Fig. 3(a–d)). However, no significant correlation was observed in the nilvadipine group (whole area: y = -0.015 x + 99.9, r = 0.43, p = 0.128; central (600): y = -0.023 x + 100.9, r = 0.40, p = 0.154; middle (1200): y = -0.015 x + 100.7, r = 0.34, p = 0.228; peripheral: y = -0.010 x + 99.8, r = 0.26, p = 0.370) (Fig. 4(a–d)). Representative images of the retina are shown in Fig. 5(a–f).
Fig. 3.
Regression analysis of the pressure insult and retinal ganglion cell (RGC) survival rate in the vehicle group. Regression analysis of whole (a), central (b), middle (c), and peripheral (d) retinal areas in the vehicle group. Linear regression analysis showed a significant negative correlation between the pressure insult and RGC survival rate (whole retina: r = 0.76, p < 0.001; central; r = 0.71, p < 0.001; middle; r = 0.78, p < 0.001; peripheral; r = 0.71, p < 0.001).
Fig. 4.
Regression analysis of the pressure insult and retinal ganglion cell (RGC) survival rate in the nilvadipine group. Regression analysis of whole (a), central (b), middle (c), and peripheral (d) retinal areas in the nilvadipine group. Linear regression analysis showed no significant correlation between the pressure insult and RGC survival rate (whole retina: r = 0.43, p = 0.128; central; r = 0.40, p = 0.154; middle; r = 0.34, p = 0.228; peripheral; r = 0.26, p = 0.370).
Fig. 5.
Retinal images of the pressure insult in laser-treated eyes. Representative retinal images of central (a), middle (b), peripheral (c), and whole (g) areas of laser-treated eyes in the vehicle group. Representative retinal images of central (d), middle (e), peripheral (f), and whole (h) areas of laser-treated eyes in the nilvadipine groups.
4. Discussion
This study successfully evaluated nilvadipine as a treatment for RGC death induced by OH. Glaucomatous optic disc damage is thought to be caused by mechanical stress and impaired blood circulation due to IOP [22]. Recently, improved IOP measurement techniques have enabled the use of mouse models, which have significant advantages in terms of handling, the application of transgenic techniques, and cost [19,23,24]. Furthermore, the development of several mouse glaucoma models has provided clues regarding the underlying causes of glaucoma [19,25,26]. Although DBA2j mice, which spontaneously develop OH, were often used in glaucoma studies, recent evidence indicates that optic nerve changes occur independent of OH and are caused by other factors, such as an immune inflammatory response [27,28]. We previously published an assessment of RGC death in a mouse model of OH using normal mouse eyes expressing CFP. Our group performed serial retinal fundus photography of a retinal ischemia reperfusion model in CFP mice and found 34.2%, 24.1%, 23.0%, and 22.2% RGC death after 1, 2, 3, and 4 weeks of treatment. In addition, thinning of the RGC layer and decreased number of RGCs were also confirmed by histological evaluation [29]. Using CFP mice, we successfully generated a laser-induced ocular hypertension mouse model showing mild high IOP, which was more closely related to human primary open-angle glaucoma and demonstrated a correlation between IOP insult and RGC damage. Histological evaluation also showed degeneration of the optic nerve due to IOP injury [20]. In this study, we examined the effect of nilvadipine on RGC death in a laser-induced ocular hypertension CFP mouse model to develop a novel drug evaluation tool that could easily screen for drugs with retinal protective effects. Since nilvadipine has been reported to improve ocular blood flow [[30], [31], [32]] and protect the retina [21,33] in many animal studies, retinal protection can be expected with this drug evaluation tool, and nilvadipine was selected as the study drug. This allowed RGCs to be identified and counted without invasive techniques such as retrograde labeling, and provided a built-in control for each mouse. Our system may be useful for evaluating the preclinical effects of neuroprotective drugs in the short term, even with a relatively small budget.
One of the challenges in experimental glaucoma models is the variation in IOP increases, and in the duration thereof, in each eye. To address this issue, we quantified the IOP increase in each eye using pressure insult values and found a negative relationship between pressure insult and RGC damage [20]. Our study supported the view that elevated IOP is a risk factor for RGC death, and that the degree of RGC damage is pressure-dependent (Fig. 3(a–d)). In contrast, despite similar pressure insults, treatment with nilvadipine reduced pressure insult-dependent RGC loss (Table 2(b) and Fig. 4(a–d)). The protective effect observed in our study strongly supports previous clinical data showing that nilvadipine inhibited visual field progression in glaucoma patients [13,14].
In addition to inhibiting pressure-induced RGC death in a mouse model, nilvadipine, a CCB, showed similar effects in a clinical study of a limited number of glaucoma patients [34]. One way to achieve this protection is to improve ocular blood flow. CCBs inhibit calcium influx into vascular smooth muscle cells, cause peripheral vasodilation, decrease vascular resistance, increase blood flow, and promote the deformability of erythrocytes. Compared with other CCBs such as nifedipine, nicardipine, and diltiazem, nilvadipine (a dihydropyridine-type CCB) is more vasoselective, i.e. it binds vascular smooth muscle more strongly than myocardium [17,35]. In addition, nilvadipine is highly selective for cerebral blood vessels [36], and improves cerebral circulation more than other CCBs [34]. Nilvadipine reaches the central nervous system in higher concentrations than other CCBs, and may increase regional cerebral blood flow [37,38]. In animal studies, CCBs increased blood flow in the choroid, retina, and optic disc [[30], [31], [32]]. In addition, there have been several reports of improved peripheral blood flow. Color Doppler imaging of the retrobulbar vessels showed significantly increased blood flow in the central retinal artery and short posterior ciliary arteries [39]. A scanning laser-Doppler flowmetry test gave similar results [40]. Nilvadipine improved the ophthalmic artery resistance index of NTG [41], decreased end diastolic velocity, and increased the optic disc flow resistance index, which would correspond to a hemifield defect in patients with open-angle glaucoma [42].
Nilvadipine not only increases blood flow, but also protects neurons by directly inhibiting excessive increases in intracellular calcium ion concentrations. Chronic OH induces structural changes in the optic nerve head microcirculation that lead to ischemia. Ischemic stress induces the apoptosis of glial cells and RGCs through an excessive increase in intracellular calcium ion concentrations. In addition, increased extracellular glutamate exacerbates apoptosis. Nilvadipine is known to inhibit calcium influx into neuronal cells exposed to glutamate, thereby inhibiting apoptosis [43].
Nilvadipine is also known to induce changes in gene expression. In Royal College of Surgeons (RCS) rats, nilvadipine protected cells by inhibiting the gene expression of apoptosis-related caspases in the retina [21,44]. Other reports indicated that nilvadipine decreased the expression of other apoptosis-related genes, such as CD 45, ErbA proto-oncogene, and JAK2, and increased fibroblast growth factor 2 (FGF2) similar to activity-regulated cytoskeleton-related proteins [[45], [46], [47]]. Furthermore, in retinal degeneration (rd) mice, nilvadipine protected neurons by modulating the expression of proteins involved in calcium metabolism, apoptosis-related proteins, cell signaling-related proteins, neurotransmitters, and caspases 3, 9, and 14, which are known to induce apoptosis [33]. Takano et al. inferred that the combination of increased synaptogyrin and FGF2, and decreased caspase-dependent apoptosis, played an important role in neuroprotection [33]. In addition, nilvadipine treatment simultaneously increased gene expression-related protein synthesis encoding growth factors and neurotrophic factors such as ciliary neurotrophic factor (CNTF) and FGF, and decreased gene expression-related protein degradation and apoptosis [48]. Since CNTF is known to delay retinal degeneration [49,50], nilvadipine treatment may protect neurons by increasing CNTF levels. Further studies are needed to identify the changes in gene expression induced by nilvadipine in our OH model.
These previous reports indicate that nilvadipine treatment may improve ocular blood flow. However, CCBs may also affect blood pressure and IOP changes related to ocular perfusion pressure; both of those factors have been linked to glaucoma [51,52]. Although blood pressure was not assessed in this study, clinical studies showed that nilvadipine significantly reduced blood pressure in patients with systemic hypertension, but did not affect normal blood pressure [16,53]. In our study, IOP did not differ between the vehicle and nilvadipine groups, in line with previous studies [31]. Therefore, nilvadipine may improve retinal blood flow without affecting ocular perfusion pressure.
Several animal models have demonstrated the benefits of nilvadipine for the treatment of retinal diseases. Nilvadipine treatment suppressed retinal damage in rat models of acute retinal ischemia [18] and cancer-related retinopathy [54], improved retinal morphology, and attenuated electroretinogram deterioration in an RCS rat model of retinitis pigmentosa [21,55]. In addition, nilvadipine treatment inhibited retinal thinning and preserved photoreceptor cells in a model of retinitis pigmentosa in rd mice [33,48]. These reports suggest that nilvadipine is delivered into the retina at effective concentrations, and is highly beneficial in the treatment of several retinal diseases. In addition, several clinical studies with CCBs have reported the potential of nilvadipine for the treatment of glaucoma [[11], [12], [13], [14]]. In this study, nilvadipine was administered intraperitoneally once daily, at doses similar to those orally administered to hypertensive human patients [56]. Furthermore, previous reports also showed that this dose of nilvadipine had a positive effect on retinal disease [21,33,48]. Although the mechanism of action of nilvadipine in terms of the neuroprotective effect observed in this study is not clear, it is inferred that the neuroprotective effect was observed by the combined effects of improving blood flow, suppressing intracellular influx of calcium and regulating the expression of apoptosis-related genes in the neuropathy induced by intraocular pressure. These results confirm that nilvadipine is effective as an add-on therapy for reducing IOP in the treatment of glaucoma. Therefore, nilvadipine is a promising neuroprotective agent for the treatment of glaucoma, particularly due to its tolerable side effect profile [57].
This study had several limitations. First, the laser-induced OH model was unbalanced between the two groups due to the difficulty of controlling the increase in IOP, similar to human moderate open-angle glaucoma. Second, immediately after laser photocoagulation, eye inflammation caused IOP fluctuations. In this study, we excluded such cases by measuring IOP 1 week after laser photocoagulation, but the IOP spikes might have occurred before the initial measurement of IOP. Such changes in IOP could occur clinically in angle-closure or exfoliation glaucoma, but ischemic optic neuropathy or inflammation may affect the outcome. Third, a minimally invasive glaucoma model needs to be established. Also, nilvadipine was administered intraperitoneally in this study. Since oral administration of nilvadipine is available, the clinical impact may differ depending on the route of administration. Fourth, retinal function was not evaluated in this study. It is possible that even if RGC death due to OH could be prevented, retinal function could not be saved. Fifth, under the same experimental conditions, both vehicle and nilvadipine groups were treated with laser photocoagulation. The laser-induced ocular hypertension CFP mouse model produced success rates of 76.7% (23 of 30) in the vehicle group and 46.7% (14 of 30) in the Nilvadipine group. Previous reports have reported that Nilvadipine did not affect IOP [14,31], and therefore administration of Nilvadipine is unlikely to affect the success of model preparation. However, the effect of nilvadipine administration on the model preparation cannot be ruled out. However, since this study was intended to evaluate the effects of IOP insult and RGC damage, the laser-treated eyes in nilvadipine group showed a high IOP of 22.0 mmHg on average, and therefore this should have no impact on the study results.
In conclusion, nilvadipine protected RGCs in a transgenic mouse OH model. This model is useful as a screening tool for drugs with retinal protective effects.
Author contribution statement
Hidekazu Tsuruga: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hiroshi Murata: Performed the experiments; Analyzed and interpreted the data.
Makoto Araie: Analyzed and interpreted the data; Conceived and designed the experiments.
Makoto Aihara: Analyzed and interpreted the data; Wrote the paper; Conceived and designed the experiments.
Funding statement
Makoto Aihara was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan [22390321].
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest’s statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank T. Saeki, T. Fujishiro and S. Nakagawa in University of Tokyo for helpful technical procedure.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e13812.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Davis B.M., Crawley L., Pahlitzsch M., Javaid F., Cordeiro M.F. Glaucoma: the retina and beyond. Acta Neuropathol. 2016;132:807–826. doi: 10.1007/s00401-016-1609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman A.L., Miglior S. Risk factors for glaucoma onset and progression. Surv. Ophthalmol. 2008;53:S3–S10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 4.The Advanced Glaucoma Intervention Study (AGIS): 7 The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am. J. Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 5.Kass M.A., Heuer D.K., Higginbotham E.J., Johnson C.A., Keltner J.L., Miller J.P., Parrish R.K., 2nd, Wilson M.R., Gordon M.O. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. ; discussion 829-730, [DOI] [PubMed] [Google Scholar]
- 6.Das A.S., Regenhardt R.W., Feske S.K., Gurol M.E. Treatment approaches to lacunar stroke. J. Stroke Cerebrovasc. Dis. 2019;28:2055–2078. doi: 10.1016/j.jstrokecerebrovasdis.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang A.E., Espay A.J. Disease modification in Parkinson's disease: current approaches, challenges, and future considerations. Mov. Disord. 2018;33:660–677. doi: 10.1002/mds.27360. [DOI] [PubMed] [Google Scholar]
- 8.Sahoo A.K., Dandapat J., Dash U.C., Kanhar S. Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer's disease. J. Ethnopharmacol. 2018;215:42–73. doi: 10.1016/j.jep.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Dhapola R., Sarma P., Medhi B., Prakash A., Reddy D.H. Recent advances in molecular pathways and therapeutic implications targeting mitochondrial dysfunction for alzheimer's disease. Mol. Neurobiol. 2022;59:535–555. doi: 10.1007/s12035-021-02612-6. [DOI] [PubMed] [Google Scholar]
- 10.Sena D.F., Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst. Rev. 2017;1:Cd006539. doi: 10.1002/14651858.CD006539.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitazawa Y., Shirai H., Go F.J. The effect of Ca2(+) -antagonist on visual field in low-tension glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 1989;227:408–412. doi: 10.1007/BF02172889. [DOI] [PubMed] [Google Scholar]
- 12.Sawada A., Kitazawa Y., Yamamoto T., Okabe I., Ichien K. Prevention of visual field defect progression with brovincamine in eyes with normal-tension glaucoma. Ophthalmology. 1996;103:283–288. doi: 10.1016/s0161-6420(96)30703-3. [DOI] [PubMed] [Google Scholar]
- 13.Netland P.A., Chaturvedi N., Dreyer E.B. Calcium channel blockers in the management of low-tension and open-angle glaucoma. Am. J. Ophthalmol. 1993;115:608–613. doi: 10.1016/s0161-6420(96)30703-3. [DOI] [PubMed] [Google Scholar]
- 14.Koseki N., Araie M., Tomidokoro A., Nagahara M., Hasegawa T., Tamaki Y., Yamamoto S. A placebo-controlled 3-year study of a calcium blocker on visual field and ocular circulation in glaucoma with low-normal pressure. Ophthalmology. 2008;115:2049–2057. doi: 10.1016/j.ophtha.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Furuta T., Shibata S., Kodama I., Yamada K. Cardiovascular effects of FR34235, a new dihydropyridine slow channel blocker, in isolated rabbit myocardium and aorta. J. Cardiovasc. Pharmacol. 1983;5:836–841. doi: 10.1097/00005344-198309000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Terakawa M., Tokuma Y., Shishido A., Noguchi H. Pharmacokinetics of nilvadipine in healthy volunteers. J. Clin. Pharmacol. 1987;27:111–117. doi: 10.1002/j.1552-4604.1987.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohtsuka M., Koibuchi Y., Sakai S., Tsujioka K., Fujiwara T., Ozaki T., Maeda K., Motoyama I., Horiai H., Ono T., et al. Effects of nilvadipine on the cardiovascular system in experimental animals. Arzneimittelforschung. 1988;38:1605–1618. https://europepmc.org/article/med/3214444 [PubMed] [Google Scholar]
- 18.Uemura A., Mizota A. Retinal concentration and protective effect against retinal ischemia of nilvadipine in rats. Eur. J. Ophthalmol. 2008;18:87–93. doi: 10.1177/112067210801800115. [DOI] [PubMed] [Google Scholar]
- 19.Aihara M., Lindsey J.D., Weinreb R.N. Experimental mouse ocular hypertension: establishment of the model. Invest. Ophthalmol. Vis. Sci. 2003;44:4314–4320. doi: 10.1167/iovs.03-0137. [DOI] [PubMed] [Google Scholar]
- 20.Tsuruga H., Murata H., Araie M., Aihara M. A model for the easy assessment of pressure-dependent damage to retinal ganglion cells using cyan fluorescent protein-expressing transgenic mice. Mol. Vis. 2012;18:2468–2478. http://www.molvis.org/molvis/v18/a259 [PMC free article] [PubMed] [Google Scholar]
- 21.Yamazaki H., Ohguro H., Maeda T., Maruyama I., Takano Y., Metoki T., Nakazawa M., Sawada H., Dezawa M. Preservation of retinal morphology and functions in royal college surgeons rat by nilvadipine, a Ca(2+) antagonist. Invest. Ophthalmol. Vis. Sci. 2002;43:919–926. https://iovs.arvojournals.org/article.aspx?articleid=2200197 [PubMed] [Google Scholar]
- 22.Clark A.F., Yorio T. Ophthalmic drug discovery. Nat. Rev. Drug Discov. 2003;2:448–459. doi: 10.1038/nrd1106. [DOI] [PubMed] [Google Scholar]
- 23.John S.W., Hagaman J.R., MacTaggart T.E., Peng L., Smithes O. Intraocular pressure in inbred mouse strains. Invest. Ophthalmol. Vis. Sci. 1997;38:249–253. https://iovs.arvojournals.org/article.aspx?articleid=2161706 [PubMed] [Google Scholar]
- 24.Danias J., Kontiola A.I., Filippopoulos T., Mittag T. Method for the noninvasive measurement of intraocular pressure in mice. Invest. Ophthalmol. Vis. Sci. 2003;44:1138–1141. doi: 10.1167/iovs.02-0553. [DOI] [PubMed] [Google Scholar]
- 25.Grozdanic S.D., Betts D.M., Sakaguchi D.S., Allbaugh R.A., Kwon Y.H., Kardon R.H. Laser-induced mouse model of chronic ocular hypertension. Invest. Ophthalmol. Vis. Sci. 2003;44:4337–4346. doi: 10.1167/iovs.03-0015. [DOI] [PubMed] [Google Scholar]
- 26.Biswas S., Wan K.H. Review of rodent hypertensive glaucoma models. Acta Ophthalmol. 2019;97:e331–e340. doi: 10.1111/aos.13983. [DOI] [PubMed] [Google Scholar]
- 27.John S.W., Smith R.S., Savinova O.V., Hawes N.L., Chang B., Turnbull D., Davisson M., Roderick T.H., Heckenlively J.R. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest. Ophthalmol. Vis. Sci. 1998;39:951–962. https://iovs.arvojournals.org/article.aspx?articleid=2161711 [PubMed] [Google Scholar]
- 28.Scholz M., Buder T., Seeber S., Adamek E., Becker C.M., Lutjen-Drecoll E. Dependency of intraocular pressure elevation and glaucomatous changes in DBA/2J and DBA/2J-Rj mice. Invest. Ophthalmol. Vis. Sci. 2008;49:613–621. doi: 10.1167/iovs.07-0745. [DOI] [PubMed] [Google Scholar]
- 29.Murata H., Aihara M., Chen Y.N., Ota T., Numaga J., Araie M. Imaging mouse retinal ganglion cells and their loss in vivo by a fundus camera in the normal and ischemia-reperfusion model. Invest. Ophthalmol. Vis. Sci. 2008;49:5546–5552. doi: 10.1167/iovs.07-1211. [DOI] [PubMed] [Google Scholar]
- 30.Harino S., Riva C.E., Petrig B.L. Intravenous nicardipine in cats increases optic nerve head but not retinal blood flow. Invest. Ophthalmol. Vis. Sci. 1992;33:2885–2890. https://iovs.arvojournals.org/article.aspx?articleid=2160731 [PubMed] [Google Scholar]
- 31.Shimazawa M., Sugiyama T., Azuma I., Araie M., Iwakura Y., Watari M., Sakai T., Hara H. Effect of lomerizine, a new Ca(2+)channel blocker, on the microcirculation in the optic nerve head in conscious rabbits: a study using a laser speckle technique. Exp. Eye Res. 1999;69:185–193. doi: 10.1006/exer.1999.0689. [DOI] [PubMed] [Google Scholar]
- 32.Tomita K., Araie M., Tamaki Y., Nagahara M., Sugiyama T. Effects of nilvadipine, a calcium antagonist, on rabbit ocular circulation and optic nerve head circulation in NTG subjects. Invest. Ophthalmol. Vis. Sci. 1999;40:1144–1151. https://iovs.arvojournals.org/article.aspx?articleid=2162090 [PubMed] [Google Scholar]
- 33.Takano Y., Ohguro H., Dezawa M., Ishikawa H., Yamazaki H., Ohguro I., Mamiya K., Metoki T., Ishikawa F., Nakazawa M. Study of drug effects of calcium channel blockers on retinal degeneration of rd mouse. Biochem. Biophys. Res. Commun. 2004;313:1015–1022. doi: 10.1016/j.bbrc.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 34.Shimamoto H., Shimamoto Y. Nilvadipine increases cerebral blood flow in elderly hypertensives: comparison with nifedipine. J. Hum. Hypertens. 1995;9:271–279. https://www.researchgate.net/publication/15603051 [PubMed] [Google Scholar]
- 35.Warltier D.C., Zyvoloski M.G., Gross G.J., Brooks H.L. Comparative actions of dihydropyridine slow channel calcium blocking agents in conscious dogs: systemic and coronary hemodynamics with and without combined beta adrenergic blockade. J. Pharmacol. Exp. Therapeut. 1984;230:367–375. https://jpet.aspetjournals.org/content/230/2/367.long [PubMed] [Google Scholar]
- 36.Tokuma Y., Fujiwara T., Noguchi H. Absorption, distribution and excretion of nilvadipine, a new dihydropyridine calcium antagonist, in rats and dogs. Xenobiotica. 1987;17:1341–1349. doi: 10.3109/00498258709047164. [DOI] [PubMed] [Google Scholar]
- 37.Murata T., Suzuki R., Higuchi T., Oshima A. Regional cerebral blood flow in the patients with depressive disorders. Keio J. Med. 2000;49(Suppl 1):A112–A113. https://europepmc.org/article/med/10750356 [PubMed] [Google Scholar]
- 38.Hanyu H., Hirao K., Shimizu S., Iwamoto T., Koizumi K., Abe K. Favourable effects of nilvadipine on cognitive function and regional cerebral blood flow on SPECT in hypertensive patients with mild cognitive impairment. Nucl. Med. Commun. 2007;28:281–287. doi: 10.1097/MNM.0b013e32804c58aa. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto T., Niwa Y., Kawakami H., Kitazawa Y. The effect of nilvadipine, a calcium-channel blocker, on the hemodynamics of retrobulbar vessels in normal-tension glaucoma. J. Glaucoma. 1998;7:301–305. https://journals.lww.com/glaucomajournal/Abstract/1998/10000/The_Effect_of_Nilvadipine_a_Calcium_Channel.2.aspx [PubMed] [Google Scholar]
- 40.Tomita G., Niwa Y., Shinohara H., Hayashi N., Yamamoto T., Kitazawa Y. Changes in optic nerve head blood flow and retrobular hemodynamics following calcium-channel blocker treatment of normal-tension glaucoma. Int. Ophthalmol. 1999;23:3–10. doi: 10.1023/a:1006423919238. [DOI] [PubMed] [Google Scholar]
- 41.Niwa Y., Yamamoto T., Harris A., Kagemann L., Kawakami H., Kitazawa Y. Relationship between the effect of carbon dioxide inhalation or nilvadipine on orbital blood flow in normal-tension glaucoma. J. Glaucoma. 2000;9:262–267. doi: 10.1097/00061198-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Rankin S.J., Drance S.M., Buckley A.R., Walman B.E. Visual field correlations with color Doppler studies in open angle glaucoma. J. Glaucoma. 1996;5:15–21. https://europepmc.org/article/med/8795729 [PubMed] [Google Scholar]
- 43.Otori Y., Kusaka S., Kawasaki A., Morimura H., Miki A., Tano Y. Protective effect of nilvadipine against glutamate neurotoxicity in purified retinal ganglion cells. Brain Res. 2003;961:213–219. doi: 10.1016/s0006-8993(02)03951-3. [DOI] [PubMed] [Google Scholar]
- 44.Katai N., Kikuchi T., Shibuki H., Kuroiwa S., Arai J., Kurokawa T., Yoshimura N. Caspaselike proteases activated in apoptotic photoreceptors of Royal College of Surgeons rats. Invest. Ophthalmol. Vis. Sci. 1999;40:1802–1807. https://iovs.arvojournals.org/article.aspx?articleid=2199735 [PubMed] [Google Scholar]
- 45.Faktorovich E.G., Steinberg R.H., Yasumura D., Matthes M.T., LaVail M.M. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347:83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- 46.Lyford G.L., Yamagata K., Kaufmann W.E., Barnes C.A., Sanders L.K., Copeland N.G., Gilbert D.J., Jenkins N.A., Lanahan A.A., Worley P.F. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 47.Sato M., Ohguro H., Ohguro I., Mamiya K., Takano Y., Yamazaki H., Metoki T., Miyagawa Y., Ishikawa F., Nakazawa M. Study of pharmacological effects of nilvadipine on RCS rat retinal degeneration by microarray analysis. Biochem. Biophys. Res. Commun. 2003;306:826–831. doi: 10.1016/s0006-291x(03)01092-1. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi K., Nakazawa M., Mizukoshi S. Systemic administration of nilvadipine delays photoreceptor degeneration of heterozygous retinal degeneration slow (rds) mouse. Exp. Eye Res. 2008;86:60–69. doi: 10.1016/j.exer.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Cayouette M., Behn D., Sendtner M., Lachapelle P.C. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J. Neurosci. 1998;18:9282–9293. doi: 10.1523/JNEUROSCI.18-22-09282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wen R., Tao W., Li Y., Sieving P.A. CNTF and retina. Prog. Retin. Eye Res. 2012;31:136–151. doi: 10.1016/j.preteyeres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leske M.C., Heijl A., Hyman L., Bengtsson B., Dong L., Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 52.Leske M.C., Wu S.Y., Hennis A., Honkanen R.B. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 53.Ogasawara K., Noda A., Yasuda S., Kobayashi M., Yukawa H., Ogawa A. Effect of calcium antagonist on cerebral blood flow and oxygen metabolism in patients with hypertension and chronic major cerebral artery occlusion: a positron emission tomography study. Nucl. Med. Commun. 2003;24:71–76. doi: 10.1097/01.mnm.0000051634.18733.46. [DOI] [PubMed] [Google Scholar]
- 54.Ohguro H., Ogawa K., Maeda T., Maruyama I., Maeda A., Takano Y., Nakazawa M. Retinal dysfunction in cancer-associated retinopathy is improved by Ca(2+) antagonist administration and dark adaptation. Invest. Ophthalmol. Vis. Sci. 2001;42:2589–2595. https://iovs.arvojournals.org/article.aspx?articleid=2200084 [PubMed] [Google Scholar]
- 55.Takano Y., Ohguro H., Ohguro I., Yamazaki H., Mamiya K., Ishikawa F., Nakazawa M. Low expression of rhodopsin kinase in pineal gland in Royal College of Surgeons rat. Curr. Eye Res. 2003;27:95–102. doi: 10.1076/ceyr.27.2.95.15953. [DOI] [PubMed] [Google Scholar]
- 56.Kuramoto K. Double-blind studies of calcium antagonists in the treatment of hypertension in Japan. J. Cardiovasc. Pharmacol. 1989;13(Suppl 1):S29–S35. doi: 10.1097/00005344-198900131-00007. [DOI] [PubMed] [Google Scholar]
- 57.Ohtsuka M., Yokota M., Kodama I., Yamada K., Shibata S. New generation dihydropyridine calcium entry blockers: in search of greater selectivity for one tissue subtype. Gen. Pharmacol. 1989;20:539–556. doi: 10.1016/0306-3623(89)90084-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.