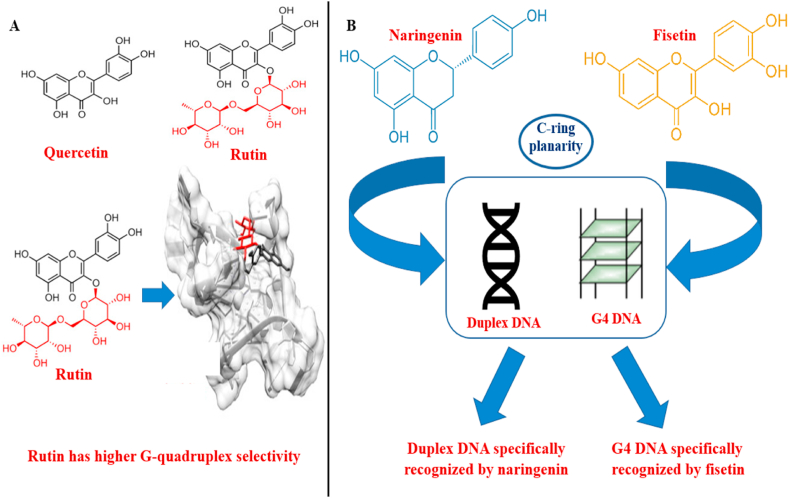
Fig. 5.
Interaction of G-quadruplex with dietary flavonoids. (A) Rutin, a flavonoid glycoside, has higher G-quadruplex selectivity than quercetin. The rutinose portion is highlighted in red. Rutin was expected to interact through stacking, the most effective strategy for G-quadruplex stabilisation, in this computational model. (B) The structural differences between naringenin and fisetin allow these compounds to interact differently with various DNA configurations. C-ring planarity seems to be a critical element in preferred G-quadruplex DNA binding of flavonoids. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)