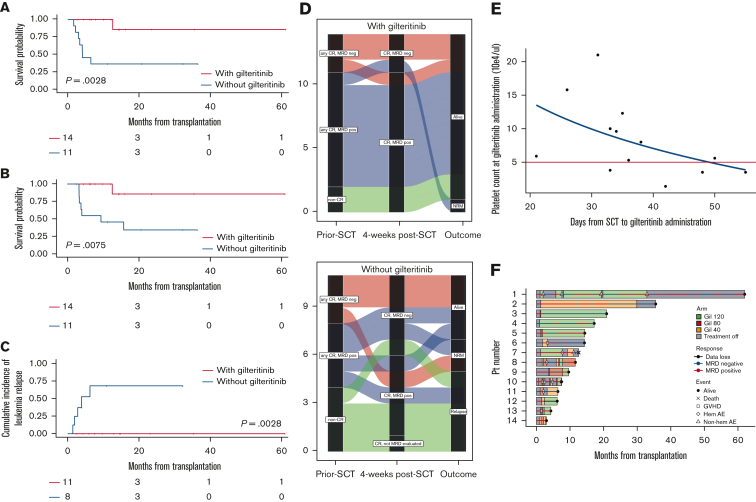
Figure 1.
Clinical impacts of early initiation of low-dose gilteritinib maintenance on posttransplant outcomes in patients with R/R FLT3mut AML. (A) RFS from transplantation (1-year RFS, 100% [95% CI, 100-100] in the gilteritinib group vs 36.4% [95% CI, 11.2-62.7] in the non-gilteritinib group; P = .0028). The median RFS in the gilteritinib and non-gilteritinib group was NR and 6.5 months (95% CI, 2.2-NR), respectively. (B) OS after transplantation (1-year OS, 100% [95% CI, 100-100] in the gilteritinib group vs 45.5% [95% CI, 16.7-70.7] in the non-gilteritinib group; P = .0075). The median OS in the gilteritinib and non-gilteritinib group was NR and 9.5 months (95% CI, 3.3-NR), respectively. (C) Cumulative relapse rate in patients with MRD-positive or non-CR before SCT (n = 19) (1-year relapse rate, 0% [95% CI, 0-0] in the gilteritinib group vs 68.8% [95% CI, 35.9-95.2] in the non-gilteritinib group, P = .0028). (D) Alluvial diagrams according to pre-SCT status, post-SCT status at week 4 (day 28-45), and outcome with gilteritinib maintenance therapy (upper panel) or not (lower panel). Each line indicates the clinical course of the patient. No patient with residual MRD or non-CR pre-SCT in the gilteritinib group (blue and green lines) relapsed, whereas 5 patients in the non-gilteritinib group (blue and green lines) relapsed. Moreover, none of the patients with MRD-positive post-SCT in the gilteritinib group relapsed, whereas 3 patients with MRD-positive post-SCT in the non-gilteritinib group relapsed. (E) Scatterplot of platelet counts during gilteritinib maintenance therapy post-SCT and days of resumption after SCT. A non-linear regression was observed. Five patients restarted gilteritinib treatment when their platelet count was less than 50 000/μL (area under the red line). One patient who resumed gilteritinib 110 days after SCT is not shown in this figure. (F) Swimmer plot of patients who received gilteritinib maintenance therapy. We observed 14 discontinuations in 7 patients (50.0%), mainly due to thrombocytopenia (n = 3), neutropenia (n = 2), anemia (n = 1), and acute GVHD (n = 2). Patient 7 died of hematolytic anemia without leukemia relapse. The cause of hemolytic anemia was considered potential graft-versus-host effect rather than gilteritinib adverse events. No other factors of hemolytic anemia, such as collagen disease, parvovirus B19, other viral reactivation or infections, secondary malignancies, Coombs test, drug, and disseminated intravascular coagulation, were detected.