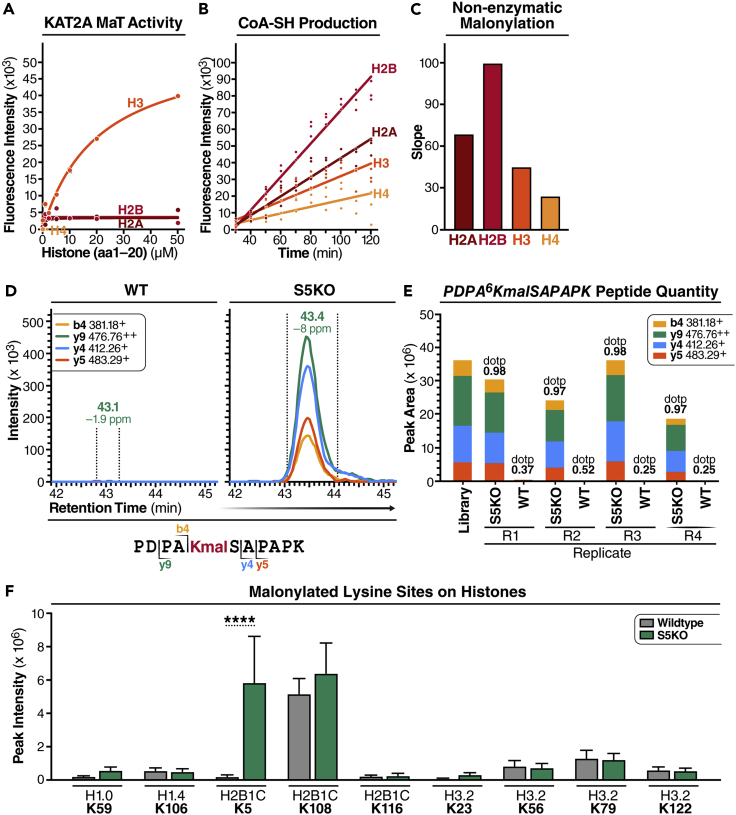
Figure 4.
Histone H2B is malonylated at K5 in SIRT5 KO mouse brain and liver
(A) In vitro lysine malonyltransferase (KMaT) activity assay of the catalytic domains of KAT2A using synthesized histone peptides (20 amino acids at N terminus) as substrates at given concentrations in the presence of 50 μM malonyl-CoA. The reactions were stopped after incubation at 37°C for 20 min. KMaT activity was evaluated by measuring CoA-SH production using CPM at Ex = 390 nm/Em = 460 nm.
(B and C) Non-enzymatic malonylation was examined by incubating synthesized histone peptides with 50 μM malonyl-CoA, and measuring the production of CoA-SH using CPM over time at Ex = 390 nm/Em = 460 nm (B). The slope was calculated to reflect the velocity of non-enzymatic of malonylation (C).
(D) Extracted ion chromatograms of PDPA6KmalSAPAPK (m/z 589.81, z = 2+) from mouse histone H2B type 2-B (Q64525) obtained by data-independent acquisition, that indicates that malonylation of K5 is increased in S5KO mouse brain tissues compared to wildtype (WT).
(E) Quantification using the peak areas of four transitions of PDPA6KmalSAPAPK (m/z 589.81, z = 2+) in four biological replicates of S5KO and WT mouse brain tissues, respectively.
(F) Malonylated lysine sites on histones were previously identified in WT and S5KO mouse livers (Nishida, et al., Mol Cell. 2015). n = 5. Error bar: mean ± SD. ∗∗∗∗p < 10−4 using Sidak multiple comparisons test following two-way ANOVA.