Summary
The importance of antibodies, particularly neutralizing antibodies, has been known for decades. When examining the immune responses against a pathogen after a vaccination or infection it is easier to measure the levels of antigen-specific antibodies than the T-cell response, but it does not give the whole picture. The levels of neutralizing antibodies are harder to determine but give a better indication of the quality of the antibody response. The induction of long-lived antibody-secreting plasma cells is crucial for a persistent humoral immune response, which has been shown for example after vaccination with the vaccinia vaccine, where antibody levels have been shown to persist for decades. With the SARS-CoV-2 pandemic ravaging the world for the past years and the monumental effort in designing and releasing novel vaccines against the virus, much effort has been put into analysing the quantity, quality, and persistence of antibody responses.
Keywords: B cells, antibodies, antigens, memory, vaccine
The importance of antigen, availability and retention, is becoming clearer. It is a crucial factor for a strong and long-lived immune response, especially after vaccination. Here we discuss recent evidence from the viewpoint of the SARS-CoV-2 pandemic versus vaccination.
Graphical Abstract
Graphical Abstract.
The current review will focus on the plasma cells and discuss how long-lasting immune responses are induced, the importance of antigen availability and retention in evoking a long-lasting persisting antibody response.
Since SARS-CoV-2 is a novel disease and many different vaccination strategies have been generated, focus will be put on persisting antibody responses against these different types of vaccines, which will give an insight into the generation of immunological memory and the persistence of antibody responses.
Inject and forget, that is how the optimal vaccine should work, a single dose, preferably needle-free which would ultimately lead to an immediate and long-lasting protection for the remainder of your life. Unfortunately, we have not yet reached that stage yet, although the advances in the field of vaccinology have been monumental since the introduction of the first vaccine, the vaccinia vaccine in the late 18th century. The first vaccines that were developed contained live, live attenuated, or inactivated/killed whole organisms. More recently, purified recombinant protein vaccines were developed, they are generally less immunogenic than their more traditional counterparts. This has driven the need for including adjuvants in modern vaccine formulations, an immune-stimulating agent that has the ability to enhance and modulate responses to antigens. Accordingly, it can be used as a tool to elicit suitable immune responses towards specific pathogens based on their particular mode of action [1]. Moreover, it is possible to tailor the vaccine itself based on the nature of the pathogen protection is needed against and what type of immune responses are needed to induce the protection. The best and most recent example is the monumental effort by the research and pharmaceutical community where they managed to come together, design, test, and release a number of different vaccines against the SARS-CoV-2 virus in a record time.
The SARS-CoV-2 pandemic has been ongoing for almost 3 years, during that time immunology, especially antibodies and concepts such as herd immunity and booster doses have become household knowledge. Moreover, vaccines and vaccinations have been a big part of public life and discussion, both scientifically and politically, for better or worse.
The vaccines against SARS-CoV-2 have been available for 2 years and most of us have received two to three doses (some even a fourth dose), which does not come close to the optimal single dose, inject, and forget strategy. Why is that?
This review will tackle that question and attempt to define and discuss the relationship between antigen load and its retention in relation to the persistence of humoral immune response, from the viewpoint of B cell induction and antibody-secreting plasma cells. We hope to shed a little light on this important relationship, especially with regard to immunological memory and persistence in response to vaccination and infection. Focus will be put on the SARS-CoV-2 vaccines and the response towards COVID-19 disease because it is a novel disease and gives us an insight into the generation of immunological memory and immunological persistence.
Plasma cells
During an immune response, B cells encounter their cognate antigen through their surface immunoglobulin (Ig) or B cell receptors (BCRs) leading to their activation and differentiation. The activated B cell can differentiate into one of the following subsets; germinal center B cell that gives rise to germinal center derived memory B cells, or plasma cells, but activated B cells can also differentiate into early memory B cells or plasma cells without having entered the germinal center (Fig. 1.) [2–4]. This encounter of cognate antigen by B cells occurs predominantly in secondary lymphoid tissues, e.g. spleen, lymph nodes, and Peyer’s patches. Lymphoid tissues are specialized to scan fluids for foreign antigens but are simultaneously supporting recruitment of lymphocytes [5]. Naive B cells express BCRs that are surface Ig of isotypes IgM and/or IgD, and lack intracellular domains for signal transduction, which is conducted by the associated molecules, Igα and Igβ [6, 3]. After a naïve B cell has recognized its cognate antigen, the BCR-antigen complex is internalized, degraded, and peptide fragments from the antigen are loaded into MHC class II molecules, which are then transported to the surface of the B cell to recruit T-cell to help at the T—B cell border or interfollicular zone in the secondary lymphoid tissues, after upregulation of the chemoattractant receptors CCR7 and EBI2 [7]. The plasma cells themselves can be divided into short-lived antibody-secreting plasma cells and long-lived plasma cells, which can survive for decades [8], maintaining high antibody levels against various antigens, given that that half-life of IgG antibodies is around 2–3 weeks, depending on isotype [9]. What determines the differentiation fate of the activated B cells is intently being researched but seems to be influenced by a variety of signals, for example from the BCRs, co-receptors, and cytokines. Several studies have demonstrated that the affinity of the BCR to the antigen is a deciding factor in determining the fate of the B cell, where BCRs with low affinity impair differentiation into plasma cells, while B cells with high-affinity BCR are more likely to differentiate into plasma cells [10, 11]. Supporting this hypothesis is a study showing that time seems to have an impact on whether a B cell should differentiate into a memory or plasma cell. It shows that during the germinal center response a temporal shift occurs, first memory B cells are generated with very few somatic hypermutations and plasma cells are generated at a later stage in the germinal centre response [12], as lower-affinity B cells are preferentially recruited into the memory B cell pool [13]. Furthermore, the strength of the plasmablast proliferation seems to be in relation to whether the B cell has high-affinity for the antigen as it leads to a stronger response than B cells with lower affinity [14]. There are, however, plasmablasts that are formed in the early phases of the response without having entered the germinal center reaction. They are primarily short-lived, localize merely within lymphoid tissues, and are defined as extrafollicular plasmablasts (reviewed in [15]). A recent study reported that the cell fate is determined early upon B cell activation and was dependent upon antigen availability, whether the activated B cell differentiated into an early extrafollicular plasmablasts, non-derived germinal center early memory B cell, or B cell that enters the germinal center reaction [16].
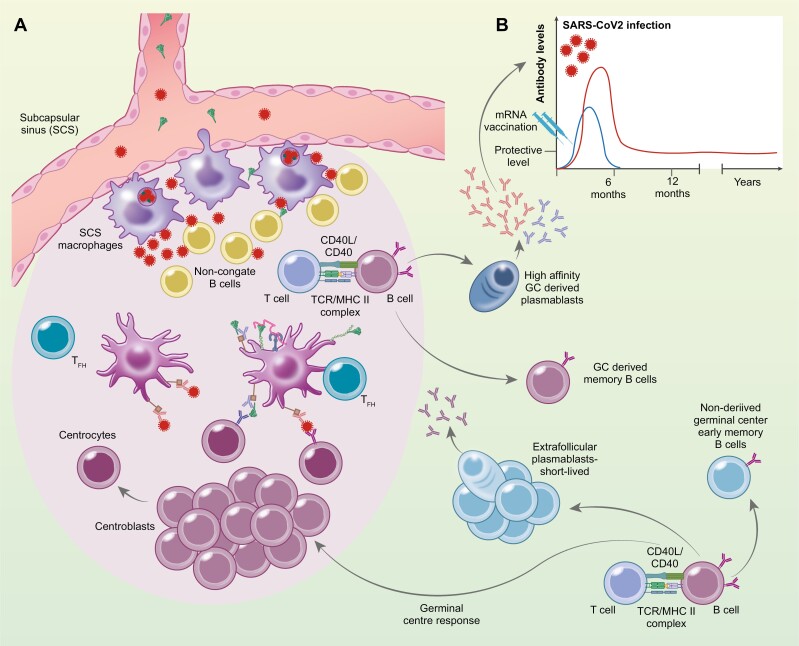
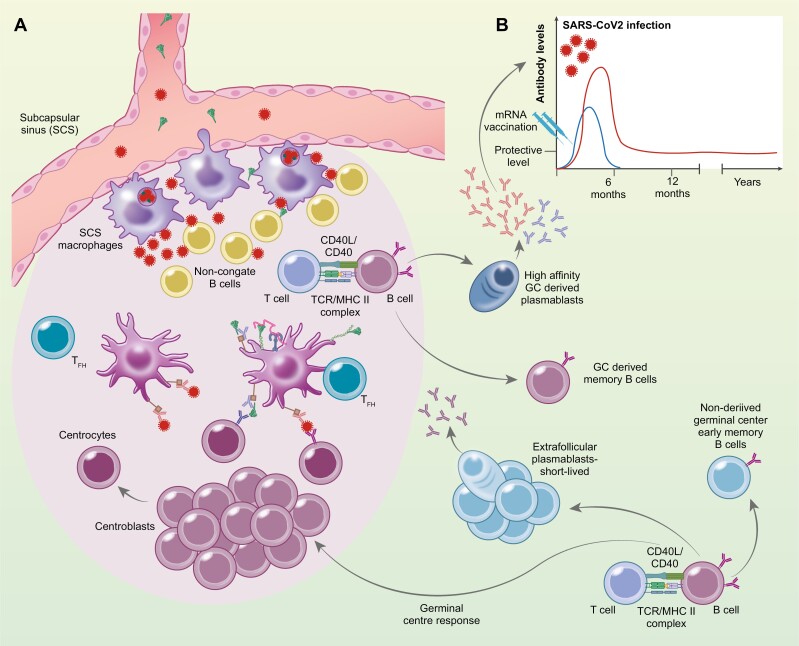
Figure 1:
Antigen delivery and retainment in draining lymph nodes. The antigens, degraded mRNA, translated Spike protein after mRNA vaccination (blue) or infection, SARS-CoV2 virus (red) reaches the lymph nodes through afferent lymph that drains into the subcapsular sinus (SCS). SCS macrophages access antigen in the lymph through cellular protrusions into the sinus. The antigen gains access to the outer cortex of the lymph nodes where it encounters non-cognate B cells that transport the antigens to the follicular dendritic cells (FDCs). SCS marcophages can also allow restricted replication of viruses inside themselves that magnifies the amount of antigen into the follicle and available for the induction, generation, and maintenance of protective B cells. The B cells require cognate antigen, retained by the FDCs in form of complexes, either as complement:Ag on complement receptors (CR) or as immunocomplexes, Ab:Ag on FcgR expressed by FDCs. The B cell consequently migrates to the border of the T and B cell zone or the interfollicular region, where they interact with antigen-specific T cells which have received T cell help (pre-T follicular helper cell (Tfh)). After this T cell help, the B cells can follow one of three alternative pathways: they become extrafollicular short-lived plasmablasts, or non-derived germinal center early memory B cells or they can enter the follicle and differentiate into centroblasts that reside in the dark zone of the germinal center (GC) and undergo clonal expansion. Some of the Tfh cells that have established stable interactions with B cells at the outer T cell zone can also enter the GC. During proliferation of centroblasts, somatic hypermutations introduce base-pair changes into the V(D)J region of the rearranged that lead to enhanced affinity for the Ag. Centroblasts then differentiate into centrocytes and move to the light zone, where the modified BCR, with help from immune helper cells, including Tfh cells and follicular dendritic cells (FDCs), select the B cells with the highest affinity for the Ag and provide them with survival signals. The centrocytes selected eventually differentiate into either memory B cells or high affinity antibody secreting cells. (B) Histogram depicts the difference in SARS-CoV-2 specific Ab persistence after mRNA vaccination (blue) vs. natural SARS-CoV-2 infection (red).
Germinal centre response
GCs are specialized microenvironments within secondary lymphoid tissues, within a network of stromal cells known as follicular dendritic cells (FDCs), in which B cells undergo extensive rounds of proliferation, somatic hypermutations, affinity-driven selection, differentiation, and isotype switching [17]. In non-active follicles, the FDCs play a vital role in organizing the B cells into these identifying clusters of the follicles (reviewed in [18]). Germinal center formation begins after the activated B cell receives help from a previously activated CD4 T cell that is specific for the same antigen or molecular complex, at the T—B cell border or interfollicular zone [19]. This interaction results in extreme B cell proliferation, located in the outer sphere of the follicle [20], leading to a formation of a cluster apart from the FDC network. This is the initial formation of the germinal center and becomes polarized into two distinct microenvironments, named the dark zone and the light zone. The light zone contains the germinal center B cells or centrocytes, T follicular helper cells, and the FDCs that carry antigen-antibody immune complexes via FcRs [21] and complement receptors [22], to present the antigens to B cells. The dark zone contains the highly proliferating germinal center B cells or centroblasts, and CXCL12-expressing reticular cells (reviewed in [23]). The centroblasts express the enzyme activation-induced cytidine deaminase (AID) and the error-prone DNA polymerase that are crucial for the initiation of somatic hypermutation in the variable region of immunoglobulin genes and are consequently vital for affinity maturation [24]. FDCs in the light zone have the unique capacity to retain immune complexes (IC) on their cell surface through previously mentioned receptors for weeks and even several months. In mice, FDCs are known to retain intact opsonized antigens for long periods of time, up to 12 months and therefore FDCs are speculated to have the ability to retain antigens for years (reviewed in [18]). This antigen retention capacity of the FDCs was believed to be due to a mechanism that protects antigens from damage [25]. It has been demonstrated that ICs undergo periodic cycling in FDCs that prolongs the half-life of the antigen [26].
The centroblasts proliferate and mutate their variable genes in the dark zone and migrate to the light zone to test their newly mutated receptors, a competition for the antigens retained on the surface of FDCs [17]. Thus, those germinal center B cells with the highest affinity for the antigen are more efficient to internalize and present it in a higher abundance of MHC class II molecules, resulting in enhanced T follicular helper cell interaction [27]. Furthermore, this enhanced interaction induces higher Myc expression that leads to positive selection of the germinal center B cells or returns to the dark zone [28] and results in the output of memory B cells and high-affinity antibody-producing plasma cells that relocate to the bone marrow and together these cells contribute to sustained protective immunity [12, 17].
In a murine model of repeated i.p. vaccinations, it has been suggested that there is a cap on the number of available GC niches, where the FDCs formed a predetermined number of clusters. Excessive occupancy of these niches suppressed responses towards new antigens [29]. Whether or not this observation can be converted over to the human setting and whether or not they have a clinical significance, remains to be determined. To put it into perspective, individuals are exposed to multiple viral, bacterial, and environmental antigens throughout their lives, and the gastrointestinal tract contains a higher number of bacteria than there are cells in the human body [30]. Clinical vaccine studies have shown that following multiple vaccinations children have a similar, and even reduced risk of infections [31–33]. These studies could indicate that even though there might be a cap on the number of available GC niches, components of the vaccine itself, such as PAMPs and being a live vaccine, and the immunological activity it confers could overcome the detrimental effects in the GC. In addition, immunization or inflammation activates the signalling pathway of Toll-like receptors in FDCs to promote GC B cell survival (Fig. 1a) [34]. Furthermore, B cells that emerge from the GC response can migrate to and take up antigen from the subcapsular macrophages and return to the GC, indicating that this process might be a response to enhance affinity maturation to adapt against pathogens that have a high mutation rate, leading to antigenic drift [35]. It has been reported inflammation that disrupts the layer of macrophages at subcapsular sinuses in lymph nodes led to a poor B cell response to a new antigen that generated lower numbers of GC B cells and PCs producing antigen-specific IgM or IgG antibodies [36]. Subcapsular sinus macrophages sample the free-floating antigens that enter the lymph through the afferent lymphatics within several minutes after administration of model antigen tracers or pathogens [37, 38]. Subcapsular sinus macrophages have relatively low phagocytic capacity, but can produce pro-inflammatory cytokines, and type 1 interferons [39]. Instead, these subcapsular sinus macrophages seem to support the replication of captured pathogens inside themselves (Fig. 1a.) [40, 43]. As it has been shown that fluorescently labelled vesicular stomatitis virus was able to replicate in wild-type lymph nodes, while mice lacking the subcapsular sinus macrophages showed no virus replication [40, 41]. This mechanism is thought to restrict viral spreading to other organs, but it can also be thought of as antigen magnification [42].
Antigen uptake
As discussed above the subcapsular macrophages are crucial for the initiation of immune responses and clearance of viruses. They surround the B cell follicles, preventing the entry of large antigens, complexes, and extracellular vesicles [44, 45]. They capture and shuttle the antigen complexes to non-cognate B cells, which transport the antigens to the follicular dendritic cells (Fig. 1a) [44, 46]. Smaller antigens are able to bypass the subcapsular sinus macrophages and enter the follicles within minutes after an injection of antigen into the bloodstream [47, 48] while larger antigens (over 70 kDa) are captured by the subcapsular sinus macrophages. The question is, what effect does the nature of these different antigens have on the immune response, especially with regard to antigen retention, antigen load, and immunological persistence.
We know that the nature of antigen is crucial for the immunological persistence of antibody response, where a T-cell independent polysaccharide can do more harm than good if the individual is primed with a T-cell dependent polysaccharide [49–51].
With the emergence of mRNA vaccines, it has been shown that the mRNA transcribed by the vaccine has a very short half-life, only a few days in human tissues [52]. It is still unknown how long the protein that is produced after the mRNA-based vaccination remains in the tissues.
Increased availability of antigens, mimicking the natural infection by repeated vaccinations with exponentially increasing the dosage, induces higher antibody levels, prolonged antigen retention, and increased numbers of Tfh and B cells in the germinal center [53]. It has been shown after repeated influenza vaccinations that the speed of waning hemagglutinin inhibition titer increases with each vaccination. Where a 2-fold decrease in the hemagglutinin inhibition titer takes 32 months for a 2-fold decrease after the first vaccination, whereas only 9 months after the seventh vaccination [54]. The authors speculate that the initial vaccination induces a broader more cross-reactive response, while repeated vaccinations induce a more targeted response that is more efficient at viral neutralization, which is not measurable by the hemagglutinin inhibition assay. Accordingly, it has been shown that antibodies from the primary response can either enhance or, conversely, restrict the GC participation of naive B cells: where broad-binding, low-affinity, and low-titer antibodies lead to enhanced recruitment of naïve B cells, but high titers of high-affinity antibodies weakened naive B cell recruitment. Thus, the intensity of the secondary response seems to be determined by the antibody concentration, affinity, and epitope specificity from previous antigen exposure [55–57]. There are several potential strategies to overcome this antibody-mediated restriction, firstly by increasing the antigen availability which can be addressed by increasing the amount of antigen administered [55], or with continuous delivery of the antigen through osmotic pumps that lead to enhanced antigen deposition—which increases the magnitude and diversity of B cell responses and resulting in higher antibody titers [53, 58].
Recently, in children suffering from Multisystem Inflammatory Syndrome in children, it has been demonstrated that the gastrointestinal tract can function as a survival niche for the SARS-CoV-2 virus, where it can reside for months after the children have overcome the infection, and if intestinal permeability breaks it can contribute to antigenemia [59]. The presence of antigens in serum can also mask the humoral responses, where seroconversion is not detected due to antigen–antibody complexes in the bloodstream. This could be one reason behind the ‘non-responders’ detected in the SARS-CoV-2 pandemic [60].
Immunological persistence after vaccinations
To this day, smallpox is still the only infectious disease infecting humans that has been eradicated by mass vaccination (rinderpest has been eradicated in cattle). It must thou be noted that not all pathogens can be eradicated, there are a number of criteria that have to be fulfilled for it to be possible and feasible to eradicate the pathogen, such as not being prone to mutations [61, 62], having effective and practical interventions available. The vaccinia vaccine is one of the most effective vaccines ever used and was an essential part of the successful eradication of smallpox. Although, it has some side effects with 1/1000 experiencing adverse reactions [63]. Vaccinia-specific antibodies have been shown to be long-lasting [64], both IgG and neutralizing antibodies can be maintained over a period of 88 years after vaccination. Accordingly, vaccinia-specific memory responses can be elicited by stimulation of both T and B cells more than 45 years after vaccination [65], and long-lived plasma cells are also detected in the bone marrow of elderly individuals that were vaccinated in childhood [66]. Furthermore, the immune response after vaccination has been shown not to be different from that of individuals who survived an active smallpox infection [67].
Another successful live attenuated vaccine is the yellow fever vaccine, where one dose induces a robust and long-lived immune response, although the antibody titers decrease over time [68].
Persisting neutralizing antibodies against measles have been shown 26–33 years after vaccination with an attenuated vaccine [69]. For the measles, mumps, and rubella vaccines seropositivity has been shown to be high (74–100%) 12–15 years after the second dose. However, with a fast and significant decline in antibody levels over the years along with a high individual variation [70, 71].
Data has shown that live attenuated vaccines lead to the induction of long-lived immune responses with persisting protective antibody titers. While subunit vaccines and toxoid vaccine induces potent immune responses, nonetheless they are not as persistent and the need for booster administrations on a more regular basis, such as for the tetanus and diphtheria vaccines [72–74].
Results have been emerging on the persistence of antibody secretion after vaccination with the novel SARS-CoV-2 mRNA and viral vector vaccines. However, whether it will be as long-lived as for the live attenuated vaccines or if it will be shorter as for the subunit and toxoid vaccines remains to be seen.
SARS-CoV-2 as a model for antibody persistence
One of the many unique characteristics of the SARS-CoV-2 pandemic is that we have a pathogen that the population, as a whole, is naive to and we can thus, on a population level, study the immune responses, generation of memory, and immunological persistence of the immune responses against the pathogen. The importance of antibodies in a protective role, especially IgG against COVID-19 disease has been supported by the efficacy of passive immunization with monoclonal antibodies against the spike protein [72, 75].
Dosage
During the pandemic, both the Pfizer-BioNTech vaccine (BNT162b2) and the Moderna (mRNA-1273) have been extensively used, and both are mRNA vaccines, where the synthetic mRNA transfects the human cells and translates the genetic information into the desired viral antigens [76]. The Oxford-AstraZeneca (ChAdOx1-S) [77] and Janssen (Ad26.COV2-S) vaccines are viral vector vaccines delivering genetic material coding for the antigen of choice into the host’s cell. Neither of the two vaccines are able to replicate. Replication competent genetically engineered vaccines have been developed and licensed, for example, the rVSV-ZEBOV vaccine against ebola [78].
Studies comparing the Pfizer and Moderna vaccines have shown that the humoral immunogenicity of the Moderna vaccine is significantly higher than of the Pfizer vaccine [79]. Which could either be explained by the 4-week interval between priming and booster for the Moderna vaccine vs. 3 weeks for the Pfizer vaccine, or the fact that the mRNA content in the Moderna vaccine is 100 μg vs. 30 μg for the Pfizer vaccine inducing a stronger priming and booster response. It should though be noted that the storage conditions of these two vaccines also play a part, as mRNA molecules are by nature unstable, the Pfizer vaccine with a lower amount of the nucleotides is stored at −80°C while Moderna with a higher amount in −20°C. Thus, the dosage can vary more between individuals vaccinated with Moderna rather than Pfizer due to mRNA degradation. One plausible explanation is that the degraded nucleotides contain adjuvant properties through activation of the intracellular TLRs that recognize nucleic acids derived from bacteria and viruses [80, 81].
Investigating the dose–response towards mRNA vaccines, it was shown that recipients who received two low doses (25 μg instead of 100 μg) of the Moderna COVID-19 vaccine showed immune memories of the virus six months after being fully vaccinated. However, two weeks after the second dose anti-spike IgG, anti-RBD IgG, and PSV neutralizing titers were about 2-fold higher in individuals receiving 100-μg when compared to those who received the 25-μg dose [82]. A dose-dependent response after the Moderna vaccine has been shown before [83, 84].
Persistence
Investigating the bone marrow of COVID-19 convalescent individuals, SARS-CoV-2 S-protein-specific plasma cells were detected in 14 out of 18 individuals, 7–8 months after infection, while none were detected in the 11 control participants [85]. The same was confirmed in vaccinated individuals, where S-protein specific plasma cells were detected in the bone marrow 6 months after vaccination with the Pfizer (BNT162b2) vaccine [86]. Was also shown in macaques that a GC response during a primary SARS-CoV-2 infection was consistent with seroconversion and was able to protect against rechallenge with a different clade of the virus [87].
When comparing the antibody levels of 234 vaccinated university employees, with the antibody levels of 65 recovered COVID-19 patients it was noted that the persistence of anti-RBD antibodies was higher 6 months after natural infection than after vaccination with the Jansen (one dose), Pfizer, or the Moderna vaccine (two doses) [88]. Blood was drawn from 49 individuals prior to SARS-CoV-2 immunization and antibody levels were measured against tetanus toxoid as an unrelated vaccine commonly used in adult immunizations and against two seasonal strains of coronaviruses. Six months after SARS-CoV-2 vaccination the IgG responses against the Pfizer and Moderna vaccines were lower than the responses against tetanus toxoid. IgG Ab levels induced by the Pfizer vaccine were lower than against the two seasonal strains of human coronaviruses, while no difference was seen between the recipients of the Moderna vaccine when compared with the two seasonal strains of coronaviruses. It should be mentioned that no difference was detected between the IgG response to the seasonal coronaviruses before or after vaccination [88].
When investigating the neutralizing efficacy of antibodies in 314 health care workers at a Swedish hospital it was shown that 90% of individuals with a verified COVID-19 infection (35/39 individuals) had detectable levels of neutralizing antibodies 200 days post-infection [89].
When examining the persistence of the antibody secreting plasma cells response. It has been shown that after more than 120 days after the second dose, the recipients of the Moderna vaccine had higher antibody levels than the Pfizer recipients [90]. Moreover, the effectiveness of the Moderna vaccine was 86% while only 75% for the Pfizer vaccine.
Correspondingly, 6 months after the second dose of the Pfizer vaccine a rapid and significant decline in antibody responses has been shown [91], while 6 months after the second dose of the Moderna vaccine the antibodies persisted [92]. Plausible explanation could be the fact as mentioned here above the high degradation properties of the mRNA molecules as the Moderna mRNA vaccine is only stored at −20°C. Thus, the degraded nucleotides could contain adjuvant properties through activation of the intracellular TLRs that recognize nucleic acids derived from bacteria and viruses and it has been shown that TLR activation in FDCs leads to enhanced survival of GC B cells, leading to more prolonged GC response and persistent antibodies. The authors would though like to note that this is just a speculation, no data is available to support this hypothesis.
Comparison between vaccines
In a prospective study investigating 288 Jordanian adults receiving two doses, 21 days apart, of either the Pfizer or the more classically designed Sinopharm (BBIBP-CorV) vaccine, an inactivated virus, it was observed that 6 weeks after the booster only 85.7% (126/147) of Sinopharm recipients were seropositive, while 99% (140/141) of individuals who received the Pfizer vaccine were seropositive. Moreover, the IgG titer was significantly higher in the Pfizer recipients when compared to the recipients of Sinopharm [93].
In a comparative study, the immune responses against 4 SARS-CoV-2 vaccines were compared six months after booster dose. Interestingly, all recipients of the Pfizer and Novovax (Nuvaxovid®), a protein-based subunit Matrix-M adjuvanted vaccine, responded with spike-protein-specific CD4 + memory T cells. Six months after vaccination the neutralization titer for the mRNA and Novovax vaccines was higher than for individuals that were naturally infected with SARS-CoV-2 [94].
The Oxford-AstraZenceca vaccine has been shown to induce high titers of IgG and neutralizing antibodies [77, 95]. When investigating the persistence of IgG antibodies it was demonstrated that anti-spike antibodies were induced by two doses of the vaccine which they persisted 6 months after vaccination [96].
Correlate of protection
In an attempt to find a correlate of protection after vaccination against COVID-19 disease, data from over 220 000 were analysed. Interestingly, it was shown that antibody levels>94 BAU/ml after two Pfizer vaccinations were associated with 67% protection while>300 BAU/ml gave 90% protection. The calculations showed that the 67% protection lasted for 2–3 months for the AstraZeneca vaccine, 5–8 months for the Pfizer vaccine, while in unvaccinated individuals after a natural infection the duration of protection was 1–2 years (Fig. 1b.) [97].
An investigation of breakthrough infections among vaccinated hospital workers showed that breakthrough infections were probably not due to waning immunity, since no difference was seen between the infected individuals and non-infected [98]. The authors speculated that the breakthrough infections might result from a lack of local protection (mucosal), shown by a sharp increase in IgA during the first day of breakthrough infection, and that the immunological memory induced by the vaccination, prevents the severe disease, but not infection. Therefore, since no local protection was present it was not able to protect against the infection [98]. Moreover, IgA class switching has been suggested to occur earlier in COVID-19 disease and higher levels during initial diseases have been associated with a worse outcome [99]. It has been shown that both the Pfizer and Moderna vaccines induce serum spike-specific IgA responses, but the levels decay significantly faster than the IgG levels [100] and whether or not it reflects the levels in the mucosal compartment and lack of protection is unknown.
Combining the results discussed above, one can make the assumption that a possible reason for the longer duration of protection after a natural infection when compared to vaccination with the mRNA and viral vector vaccines, might be that the natural infection occurs in the mucosal compartment, and thus induces mucosal immunity and gives a more potent protection against subsequent infections.
Moreover, systemic vaccination with attenuated viruses has been shown to induce mucosal immune responses [101], it has also been shown that mucosal immunization in one mucosal locale induces mucosal responses at other mucosal sites [102, 103]. Thus, a mucosal vaccine should be the best choice for a vaccine? However, it is not that simple. Even though mucosal vaccines have been in development for decades, there is only one licensed intranasal vaccine (FluMist) and eight licensed oral vaccines (inactivated and live attenuated) (reviewed in [104]). For COVID-19, it has been shown that in severe COVID-19 disease, the architecture of the immune system of the gut is compromised, impairing the ability to mount an intestinal immune response. Therefore it is crucial that the recipient is free from COVID-19 disease, when receiving the vaccine, especially for a mucosal vaccine [105].
Conclusion
To summarize, one of the factors for a waning immune response is an antigen shortage. The antigen is a crucial factor of a potent GC induction and its maintenance leads to a strong persisting immune response. Second, to not only protect against severe disease but to prevent possible breakthrough infections in vaccinated subjects, the vaccination needs to induce an immune response at the site of pathogen entry, as for SARS-CoV-2, in the mucosal lymphoid tissues.
Thus, for an inject and forget; immunization strategy to be possible three criteria have to be fulfilled for the vaccine of choice;
(i) The vaccine needs to induce immune responses at the site of entry. For SARS-CoV-2, in the mucosa, either as a strong systemic response that is also able to evoke a mucosal response or delivered as an oral or nasal vaccine.
(ii) The vaccine needs to retain its antigen long-term, in order for that to work, a live attenuated vaccine or a self-replicating viral vector would need to be designed.
(iii) A potent adjuvant would be needed, especially if the choice would be a self-replicating viral vector, preferably an adjuvant that directs the immune response towards a mucosal response.
These suggestions do not nearly cover the overwhelming complexities researchers have to tackle to design, test, and release an optimal vaccine, but it might be food for thought. During the SARS-CoV-2 pandemic, we have seen a rise in public awareness of the importance of vaccines and vaccine research. Unfortunately, we have also seen much misinformation and anti-vaccination propaganda. This puts a lot of pressure on researchers working in the field of vaccinology to inform the public in a calm, clear, and concise manner about the importance of vaccines and vaccinations.
At the end of the day, the purpose of vaccination is to induce a long-lasting protective immune response. There will be calor, dolor, rubor, and tumour, but the question that still remains to be answered by the scientific community is quite simple: how much is enough?
Acknowledgments
The authors would like to thank Hildur Sigurgrimsdóttir, M.Sc., Department of Immunology, Landspitali, for constructive comments.
Glossary
Abbreviations
- BAU
Binding antibody units
- BCR
B cell receptor
- COVID-19
coronavirus disease 2019
- FcR
fragment crystallizable receptor
- FDC
follicular dendritic cell
- GC
germinal center
- Ig
Immunoglobulin
- MHC
major histocompatibility complex
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2.
Contributor Information
Stefania P Bjarnarson, Department of Immunology, Landspitali—The National University Hospital of Iceland, Reykjavik, Iceland; Faculty of Medicine, Biomedical Center, University of Iceland, Reykjavik, Iceland.
Siggeir F Brynjolfsson, Department of Immunology, Landspitali—The National University Hospital of Iceland, Reykjavik, Iceland; Faculty of Medicine, Biomedical Center, University of Iceland, Reykjavik, Iceland.
Ethical approval
The animal research adheres to the ARRIVE guidelines (https://arriveguidelines.org/arrive-guidelines)
Conflict of Interest
The authors have no relevant financial or non-financial competing interests to declare.
Funding
The authors did not receive any grant from any funding agency for writing this manuscript.
Author contributions
Siggeir F. Brynjólfsson and Stefanía P. Bjarnarson conceived of the presented idea, wrote, and approved the final version of the article.
References
- 1. Del Giudice G, Rappuoli R, Didierlaurent AM.. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin Immunol 2018, 39, 14–21. doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 2. Yoshida T, Mei H, Dörner T, Hiepe F, Radbruch A, Fillatreau S, et al. Memory B and memory plasma cells. Immunol Rev 2010, 237, 117–39. doi: 10.1111/j.1600-065X.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- 3. Taylor JJ, Pape KA, Steach HR, Jenkins MK.. Humoral immunity. Apoptosis and antigen affinity limit effector cell differentiation of a single naïve B cell. Science 2015, 347, 784–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor JJ, Pape KA, Jenkins MK.. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med 2012, 209, 597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol 2010, 11, 989–96. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 6. Yang J, Reth M.. Receptor dissociation and B-cell activation. Curr Top Microbiol Immunol 2016, 393, 27–43. doi: 10.1007/82_2015_482. [DOI] [PubMed] [Google Scholar]
- 7. Cyster JG, Dang EV, Reboldi A, Yi T.. 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol 2014, 14, 731–43. doi: 10.1038/nri3755. [DOI] [PubMed] [Google Scholar]
- 8. Landsverk OJB, Snir O, Casado RB, Richter L, Mold JE, Réu P, et al. Antibody-secreting plasma cells persist for decades in human intestine. J Exp Med 2017, 214, 309–17. doi: 10.1084/jem.20161590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mankarious S, Lee M, Fischer S, Pyun KH, Ochs HD, Oxelius VA, et al. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med 1988, 112, 634–40. [PubMed] [Google Scholar]
- 10. Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R.. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med 2006, 203, 1081–91. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith KG, Light A, Nossal GJ, Tarlinton DM.. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J 1997, 16, 2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ.. A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity 2016, 44, 116–30. doi: 10.1016/j.immuni.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shinnakasu R, Inoue T, Kometani K, Moriyama S, Adachi Y, Nakayama M, et al. Regulated selection of germinal-center cells into the memory B cell compartment. Nat Immunol 2016, 17, 861–9. doi: 10.1038/ni.3460. [DOI] [PubMed] [Google Scholar]
- 14. Kräutler NJ, Suan D, Butt D, Bourne K, Hermes JR, Chan TD, et al. Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J Exp Med 2017, 214, 1259–67. doi: 10.1084/jem.20161533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM.. The generation of antibody-secreting plasma cells. Nat Rev Immunol 2015, 15, 160–71. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 16. Glaros V, Rauschmeier R, Artemov AV, Reinhardt A, Ols S, Emmanouilidi A, et al. Limited access to antigen drives generation of early B cell memory while restraining the plasmablast response. Immunity 2021, 54, 2005–23.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allen CDC, Okada T, Cyster JG.. Germinal-center organization and cellular dynamics. Immunity 2007, 27, 190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heesters BA, Myers RC, Carroll MC.. Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol 2014, 14, 495–504. doi: 10.1038/nri3689. [DOI] [PubMed] [Google Scholar]
- 19. Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG.. Follicular helper T cells: lineage and location. Immunity 2009, 30, 324–35. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coffey F, Alabyev B, Manser T.. Initial clonal expansion of germinal center B cells takes place at the Perimeter of Follicles. Immunity 2009, 30, 599–609. doi: 10.1016/j.immuni.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qin D, Wu J, Vora KA, Ravetch JV, Szakal AK, Manser T, et al. Fcγ receptor IIB on follicular dendritic cells regulates the B cell recall response. J Immunol 2000, 164, 6268–75. doi: 10.4049/jimmunol.164.12.6268. [DOI] [PubMed] [Google Scholar]
- 22. Ehrenstein MR, O’Keefe TL, Davies SL, Neuberger MS.. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc Natl Acad Sci USA 1998, 95, 10089–93. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Victora GD, Nussenzweig MC.. Germinal centers. Annu Rev Immunol 2022, 40, 413–42. doi: 10.1146/annurev-immunol-120419-022408. [DOI] [PubMed] [Google Scholar]
- 24. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T.. Pillars article: class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme.. 2000. 102: 553-563. J Immunol 2018, 201, 2530–40. [PubMed] [Google Scholar]
- 25. McCloskey ML, de Lafaille MAC, Carroll MC, Erlebacher A.. Acquisition and presentation of follicular dendritic cell-bound antigen by lymph node-resident dendritic cells. J Exp Med 2011, 208, 135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arulraj T, Binder SC, Meyer-Hermann M.. Rate of immune complex cycling in follicular dendritic cells determines the extent of protecting antigen integrity and availability to germinal center B cells. J Immunol 2021, 206, 1436–42. doi: 10.4049/jimmunol.2001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abbott RK, Lee JH, Menis S, Skog P, Rossi M, Ota T, et al. Precursor frequency and affinity determine B cell competitive fitness in germinal centers, tested with germline-targeting HIV vaccine immunogens. Immunity 2018, 48, 133–146.e6. doi: 10.1016/j.immuni.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dominguez-Sola D, Victora GD, Ying CY, Phan RT, Saito M, Nussenzweig MC, et al. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol 2012, 13, 1083–91. doi: 10.1038/ni.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Avancena P, Song T, Yao Y, Fehlner-Peach H, Diamond B, Gu H, et al. The magnitude of germinal center reactions is restricted by a fixed number of preexisting niches. Proc Natl Acad Sci USA 2021, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaper JB, Sperandio V.. Bacterial cell-to-cell dignaling in the gastrointestinal tract. Infect Immun 2005, 73, 3197–209. doi: 10.1128/IAI.73.6.3197-3209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aaby P, Samb B, Simondon F, Seck AMC, Knudsen K, Whittle H.. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ 1995, 311, 481–5. doi: 10.1136/bmj.311.7003.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Griffin MR, Taylor JA, Daugherty JR, Ray WA.. No increased risk for invasive bacterial infection found following diphtheria-tetanus-pertussis immunization. Pediatrics 1992, 89, 640–2. [PubMed] [Google Scholar]
- 33. Otto S, Mahner B, Kadow I, Beck JF, Wiersbitzky SKW, Bruns R.. General non-specific morbidity is reduced after vaccination within the third month of life—the Greifswald study. J Infect 2000, 41, 172–5. doi: 10.1053/jinf.2000.0718. [DOI] [PubMed] [Google Scholar]
- 34. Garin A, Meyer-Hermann M, Contie M, Figge MT, Buatois V, Gunzer M, et al. Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity 2010, 33, 84–95. doi: 10.1016/j.immuni.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y, Garcia-Ibanez L, Ulbricht C, Lok LSC, Pike JA, Mueller-Winkler J, et al. Recycling of memory B cells between germinal center and lymph node subcapsular sinus supports affinity maturation to antigenic drift. Nat Commun 2022, 13, 2460. doi: 10.1038/s41467-022-29978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaya M, Castello A, Montaner B, Rogers N, Reis e Sousa C, Bruckbauer A, et al. Host response. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science 2015, 347, 667–72. doi: 10.1126/science.aaa1300. [DOI] [PubMed] [Google Scholar]
- 37. Kuka M, Iannacone M.. The role of lymph node sinus macrophages in host defense. Ann N Y Acad Sci 2014, 1319, 38–46. doi: 10.1111/nyas.12387. [DOI] [PubMed] [Google Scholar]
- 38. Gray EE, Cyster JG.. Lymph node macrophages. J Innate Immun 2012, 4, 424–36. doi: 10.1159/000337007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Phan TG, Green JA, Gray EE, Xu Y, Cyster JG.. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol 2009, 10, 786–93. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iannacone M, Moseman EA, Tonti E, Bosurgi L, Junt T, Henrickson SE, et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature 2010, 465, 1079–83. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Farrell HE, Davis-Poynter N, Bruce K, Lawler C, Dolken L, Mach M, et al. Lymph node macrophages restrict murine cytomegalovirus dissemination. J Virol 2015, 89, 7147–58. doi: 10.1128/JVI.00480-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Honke N, Shaabani N, Cadeddu G, Sorg UR, Zhang DE, Trilling M, et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol 2011, 13, 51–7. doi: 10.1038/ni.2169. [DOI] [PubMed] [Google Scholar]
- 43. Moseman EA, Ashley Moseman E, Iannacone M, Bosurgi L, Tonti E, Chevrier N, et al. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity 2012, 36, 415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Phan TG, Green JA, Gray EE, Xu Y, Cyster JG.. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol 2009, 10, 786–93. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 2016, 352, 242–6. doi: 10.1126/science.aaf1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 2007, 450, 110–4. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 47. Pape KA, Catron DM, Itano AA, Jenkins MK.. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity 2007, 26, 491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 48. Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 2009, 30, 264–76. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Micoli F, Bjarnarson SP, Arcuri M, Aradottir Pind AA, Magnusdottir GJ, Necchi F, et al. Short Vi-polysaccharide abrogates T-independent immune response and hyporesponsiveness elicited by long Vi-CRM conjugate vaccine. Proc Natl Acad Sci USA 2020, 117, 24443–9. doi: 10.1073/pnas.2005857117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bjarnarson SP, Benonisson H, Del Giudice G, Jonsdottir I.. Pneumococcal polysaccharide abrogates conjugate-induced germinal center reaction and depletes antibody secreting cell pool, causing hyporesponsiveness. PLoS One 2013, 8, e72588. doi: 10.1371/journal.pone.0072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brynjolfsson SF, Henneken M, Bjarnarson SP, Mori E, Del Giudice G, Jonsdottir I.. Hyporesponsiveness following booster immunization with bacterial polysaccharides is caused by apoptosis of memory B cells. J Infect Dis 2012, 205, 422–30. doi: 10.1093/infdis/jir750. [DOI] [PubMed] [Google Scholar]
- 52. Probst J, Weide B, Scheel B, Pichler BJ, Hoerr I, Rammensee HG, et al. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther 2007, 14, 1175–80. doi: 10.1038/sj.gt.3302964. [DOI] [PubMed] [Google Scholar]
- 53. Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci USA 2016, 113, E6639–48. doi: 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zelner J, Petrie JG, Trangucci R, Martin ET, Monto AS.. Effects of sequential influenza A(H1N1)pdm09 vaccination on antibody waning. J Infect Dis 2019, 220, 12–9. doi: 10.1093/infdis/jiz055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tas JMJ, Koo JH, Lin YC, Xie Z, Steichen JM, Jackson AM, et al. Antibodies from primary humoral responses modulate the recruitment of naive B cells during secondary responses. Immunity 2022, 55, 1856–71. doi: 10.1016/j.immuni.2022.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bergstrom JJ, Xu H, Heyman B.. Epitope-specific suppression of IgG responses by passively administered specific IgG: evidence of epitope masking. Front Immunol 2017, 8, 238. doi: 10.3389/fimmu.2017.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang Y, Meyer-Hermann M, George LA, Figge MT, Khan M, Goodall M, et al. Germinal center B cells govern their own fate via antibody feedback. J Exp Med 2013, 210, 457–64. doi: 10.1084/jem.20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu JK, Crampton JC, Cupo A, Ketas T, van Gils MJ, Sliepen K, et al. Murine antibody responses to cleaved soluble HIV-1 envelope trimers are highly restricted in specificity. J Virol 2015, 89, 10383–98. doi: 10.1128/JVI.01653-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yonker LM, Gilboa T, Ogata AF, Senussi Y, Lazarovits R, Boribong BP, et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest 2021, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Belogiannis K, Florou VA, Fragkou PC, Ferous S, Chatzis L, Polyzou A, et al. SARS-CoV-2 antigenemia as a confounding factor in immunodiagnostic assays: a case study. Viruses 2021, 13, 1143. doi: 10.3390/v13061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hopkins DR. Disease eradication. N Engl J Med 2013, 368, 54–63. doi: 10.1056/nejmra1200391. [DOI] [PubMed] [Google Scholar]
- 62. Hopkins DR. Princes and Peasants: Smallpox in History, 1983. [Google Scholar]
- 63. Control CoD. Side Effects of Vaccinations , 2017.
- 64. Pütz MM, Alberini I, Midgley CM, Manini I, Montomoli E, Smith GL.. Prevalence of antibodies to Vaccinia virus after smallpox vaccination in Italy. J Gen Virol 2005, 86, 2955–60. doi: 10.1099/vir.0.81265-0. [DOI] [PubMed] [Google Scholar]
- 65. Halldórsdóttir H. Long Term Immunological Memory to Vaccinia Virus, Faculty of Medicine. Reykjavík, Iceland: University of Iceland, 2012:102. [Google Scholar]
- 66. Brynjolfsson SF, Mohaddes M, Kärrholm J, Wick M-J.. Long-lived plasma cells in human bone marrow can be either CD19 or CD19. Blood Adv 2017, 1, 835–8. doi: 10.1182/bloodadvances.2017004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Taub DD, Ershler WB, Janowski M, Artz A, Key ML, McKelvey J, et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med 2008, 121, 1058–64. doi: 10.1016/j.amjmed.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lindsey NP, Horiuchi KA, Fulton C, Panella AJ, Kosoy OI, Velez JO, et al. Persistence of yellow fever virus-specific neutralizing antibodies after vaccination among US travellers. J Travel Med 2018, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dine MS, Hutchins SS, Thomas A, Williams I, Bellini WJ, Redd SC.. Persistence of vaccine-induced antibody to measles 26–33 years after vaccination. J Infect Dis 2004, 189, S123–30. doi: 10.1086/380308. [DOI] [PubMed] [Google Scholar]
- 70. Davidkin I, Jokinen S, Broman M, Leinikki P, Peltola H.. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis 2008, 197, 950–6. doi: 10.1086/528993. [DOI] [PubMed] [Google Scholar]
- 71. Seagle EE, Bednarczyk RA, Hill T, Fiebelkorn AP, Hickman CJ, Icenogle JP, et al. Measles, mumps, and rubella antibody patterns of persistence and rate of decline following the second dose of the MMR vaccine. Vaccine 2018, 36, 818–26. doi: 10.1016/j.vaccine.2017.12.075. [DOI] [PubMed] [Google Scholar]
- 72. Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021, 384, 238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grasse M, Meryk A, Schirmer M, Grubeck-Loebenstein B, Weinberger B.. Booster vaccination against tetanus and diphtheria: insufficient protection against diphtheria in young and elderly adults. Immunity Ageing 2016, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. World Health O. The Immunological Basis for Immunization Series: Module 3: Tetanus. Update 2018 Edn. Geneva: World Health Organization, 2018. [Google Scholar]
- 75. Razonable RR, Pawlowski C, O’Horo JC, Arndt LL, Arndt R, Bierle DM, et al. Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine 2021, 40, 101102. doi: 10.1016/j.eclinm.2021.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pardi N, Hogan MJ, Porter FW, Weissman D.. mRNA vaccines–a new era in vaccinology. Nat Rev Drug Discovery 2018, 17, 261–79. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al.; Oxford COVID Vaccine Trial Group. Oxford CVTG. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Medaglini D, Harandi AM, Ottenhoff THM, Siegrist C-A, Consortium V-E, Agnandji ST, et al. Ebola vaccine R&D: filling the knowledge gaps. Sci Transl Med 2015, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L.. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–5. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK, et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med 2011, 208, 2357–66. doi: 10.1084/jem.20111171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martinez-Gil L, Goff PH, Hai R, Garcia-Sastre A, Shaw ML, Palese P.. A Sendai virus-derived RNA agonist of RIG-I as a virus vaccine adjuvant. J Virol 2013, 87, 1290–300. doi: 10.1128/JVI.02338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mateus J, Dan JM, Zhang Z, Rydyznski Moderbacher C, Lammers M, Goodwin B, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 2021, 374, eabj9853. doi: 10.1126/science.abj9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med 2020, 383, 1920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020, 383, 2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Turner JS, Kim W, Kalaidina E, Goss CW, Rauseo AM, Schmitz AJ, et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021, 595, 421–5. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 86. Kim W, Zhou JQ, Horvath SC, Schmitz AJ, Sturtz AJ, Lei T, et al. Germinal centre-driven maturation of B cell response to mRNA vaccination. Nature 2022, 604, 141–5. doi: 10.1038/s41586-022-04527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kim G, Kim DH, Oh H, Bae S, Kwon J, Kim M-J, et al. Germinal center-induced immunity is correlated with protection against SARS-CoV-2 reinfection but not lung damage. J Infect Dis 2021, 224, 1861–72. doi: 10.1093/infdis/jiab535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Keshavarz B, Richards NE, Workman LJ, Patel J, Muehling LM, Canderan G, et al. Trajectory of IgG to SARS-CoV-2 after vaccination with BNT162b2 or mRNA-1273 in an employee cohort and comparison with natural infection. Front Immunol 2022, 13, 850987. doi: 10.3389/fimmu.2022.850987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Marklund E, Leach S, Nyström K, Lundgren A, Liljeqvist J-A, Nilsson S, et al. Longitudinal follow up of immune responses to SARS-CoV-2 in health care workers in Sweden with several different commercial IgG-assays, measurement of neutralizing antibodies and CD4 T-cell responses. Front Immunol 2021, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bajema KL, Dahl RM, Evener SL, Prill MM, Rodriguez-Barradas MC, Marconi VC, et al.; Surveillance Platform for E, Respiratory Infectious Organisms at the VAC-SG. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans–five veterans affairs medical centers, United States, February 1–September 30, 2021. MMWR Morb Mortal Wkly Rep 2021, 70, 1700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021, 385, e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med 2021, 384, 2259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Alqassieh R, Suleiman A, Abu-Halaweh S, Santarisi A, Shatnawi O, Shdaifat L, et al. Pfizer-BioNTech and sinopharm: a comparative study on post-vaccination antibody titers. Vaccines 2021, 9, 1223. doi: 10.3390/vaccines9111223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang Z, Mateus J, Coelho CH, Dan JM, Moderbacher CR, Gálvez RI, et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–51. doi: 10.1016/j.cell.2022.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chau NVV, Nguyet LA, Truong NT, Toan LM, Dung NT, Hung LM, et al.; Oxford University Clinical Research Unit C-RG. Immunogenicity of Oxford-AstraZeneca COVID-19 vaccine in Vietnamese health-care workers. Am J Trop Med Hyg 2022, 106, 556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Robertson LJ, Price R, Moore JS, Curry G, Farnan J, Black A, et al. IgG antibody production and persistence to 6 months following SARS-CoV-2 vaccination: a Northern Ireland observational study. Vaccine 2022, 40, 2535–9. doi: 10.1016/j.vaccine.2022.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wei J, Pouwels KB, Stoesser N.et al., team C-IS. SARS-CoV-2 anti-spike IgG antibody responses after second dose of ChAdOx1 or BNT162b2 and correlates of protection in the UK general population. doi: 10.1101/2021.09.13.21263487. [DOI]
- 98. Terreri S, Piano Mortari E, Vinci MR, Russo C, Alteri C, Albano C, et al. Persistent B cell memory after SARS-CoV-2 vaccination is functional during breakthrough infections. Cell Host Microbe 2022, 30, 400–408.e4. doi: 10.1016/j.chom.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brynjolfsson SF, Sigurgrimsdottir H, Einarsdottir ED, Bjornsdottir GA, Armannsdottir B, Baldvinsdottir GE, et al. Detailed multiplex analysis of SARS-CoV-2 specific antibodies in COVID-19 disease. Front Immunol 2021, 12, 695230. doi: 10.3389/fimmu.2021.695230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wisnewski AV, Campillo Luna J, Redlich CA.. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS One 2021, 16, e0249499. doi: 10.1371/journal.pone.0249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Simon JK, Ramirez K, Cuberos L, Campbell JD, Viret JF, Muñoz A, et al. Mucosal IgA responses in healthy adult volunteers following intranasal spray delivery of a live attenuated measles vaccine. Clin Vaccine Immunol 2011, 18, 355–61. doi: 10.1128/cvi.00354-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Johansson EL, Wassén L, Holmgren J, Jertborn M, Rudin A.. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect Immun 2001, 69, 7481–6. doi: 10.1128/IAI.69.12.7481-7486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rudin A. Vaccination of humans intranasally with cholera toxin B-subunit induces antibody responses in vaginal and nasal secretions as well as in serum. Immunol Lett 1997, 56, 176. doi: 10.1016/s0165-2478(97)87544-x. [DOI] [Google Scholar]
- 104. Lavelle EC, Ward RW.. Mucosal vaccines—fortifying the frontiers. Nat Rev Immunol 2022, 22, 236–50. doi: 10.1038/s41577-021-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trevelin SC, Pickering S, Todd K.et al.Disrupted Peyer’s patch microanatomy in COVID-19 including germinal centre atrophy independent of local virus. [DOI] [PMC free article] [PubMed]