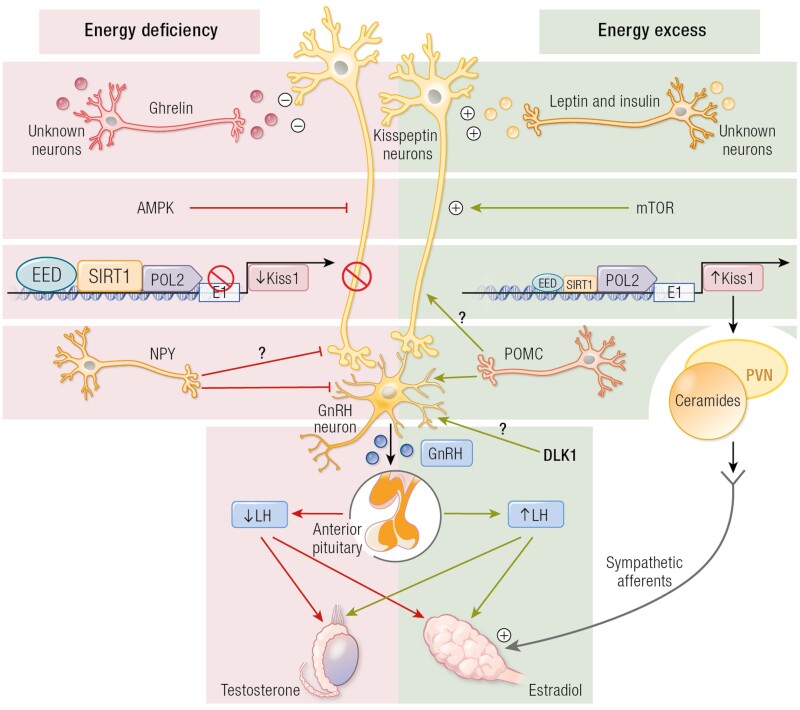
Figure 1.
Schematic representation of the putative role of cellular energy sensors and metabolic mediators in the control of puberty. In conditions of energy insufficiency, hypothalamic activation of AMP-activated protein kinase (AMPK), together with persistence of the repressive action of SIRT1 at the Kiss1 promoter, leads to reduced Kiss1 expression and delayed puberty. By contrast, in conditions of energy sufficiency and timely removal of SIRT1 from the Kiss1 promoter, together with the presumable activation of mammalian target of rapamycin (mTOR), allows increased Kiss1 expression and the normal occurrence of puberty. At extreme conditions of energy excess (eg, early-onset obesity), precocious removal of SIRT1 from the Kiss1 promoter causes a change in the chromatin landscape that accelerates the rise in Kiss1 expression, leading to early puberty. In addition, obesity presumably also causes the activation of an alternative pathway, involving kisspeptins innervation of the paraventricular nucleus (PVN) and ceramide (CER) synthesis at this site, which apparently contribute to the precocious activation of the ovary in obesity via direct sympathetic inputs. ARC, arcuate nucleus; Dyn, dynorphin; GnRH, gonadotrophin-releasing hormone; Kp, kisspeptins; mTOR, mammalian target of rapamycin; NKB, neurokinin B; SIRT1, Sirtuin; SNS, sympathetic nervous system.