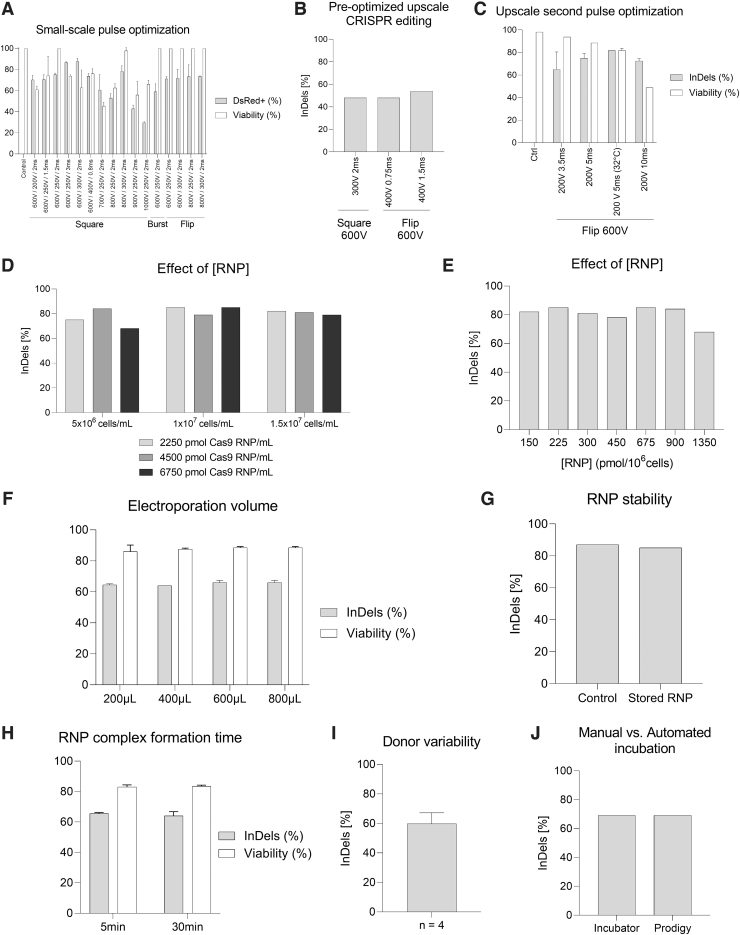
FIG. 1.
Study and optimization of electroporation conditions using CliniMACS Prodigy electroporator. (A) Small-scale pulse optimization using DsRed mRNA to determine the transfection efficiency (gray) and viability (white), n = 2. (B) Upscale BCL11A transfection using two of the most suitable electroporation settings in small-scale optimization led to moderate InDel efficiencies, n = 1. (C) Upscale comparison of different conditions to further optimize transfection efficiency. BCL11A editing is provided in InDel rate after Sanger sequencing of the PCR product (gray). Viability was determined by flow cytometry 2 days post-transfection (white), n = 2. Nonsignificant differences were observed for editing efficiencies in Mann–Whitney tests (p > 0.05). (D) Influence of different RNP concentrations (2250 pmol/mL, light gray; 4500 pmol/mL, dark gray; 6750 pmol/mL, black) with increasing cell concentrations (5 × 106 to 1.5 × 107 cells/mL) on the BCL11A editing rate, n = 1. (E) Same data as in (D), but displayed as editing rate in relation to RNP concentration per 106 cells, n = 1. (F) Effect of the electroporation volume in CliniMACS Prodigy EP-2 cuvette on the editing performance (gray) and viability (white), n = 2. Nonsignificant differences were observed for editing efficiencies and viabilities in Mann–Whitney tests (p > 0.05). (G) RNP stability controlling freshly prepared RNP (control) versus RNP recovered after a process run and storage time of 60 min, n = 1. (H) Effect of the RNP incubation time on the editing rate (light gray) and viability (white), n = 2. Nonsignificant differences were observed for editing efficiencies and viabilities in Mann–Whitney tests (p > 0.05). (I) Average editing rate for thawed HSPCs from different donors, n = 4. (J) Comparison of BCL11A transfection efficiency after cultivation in the CliniMACS Prodigy system versus a classical cell incubator, n = 1. HSPCs, hematopoietic stem and progenitor cells; PCR, polymerase chain reaction; RNP, ribonucleoprotein.