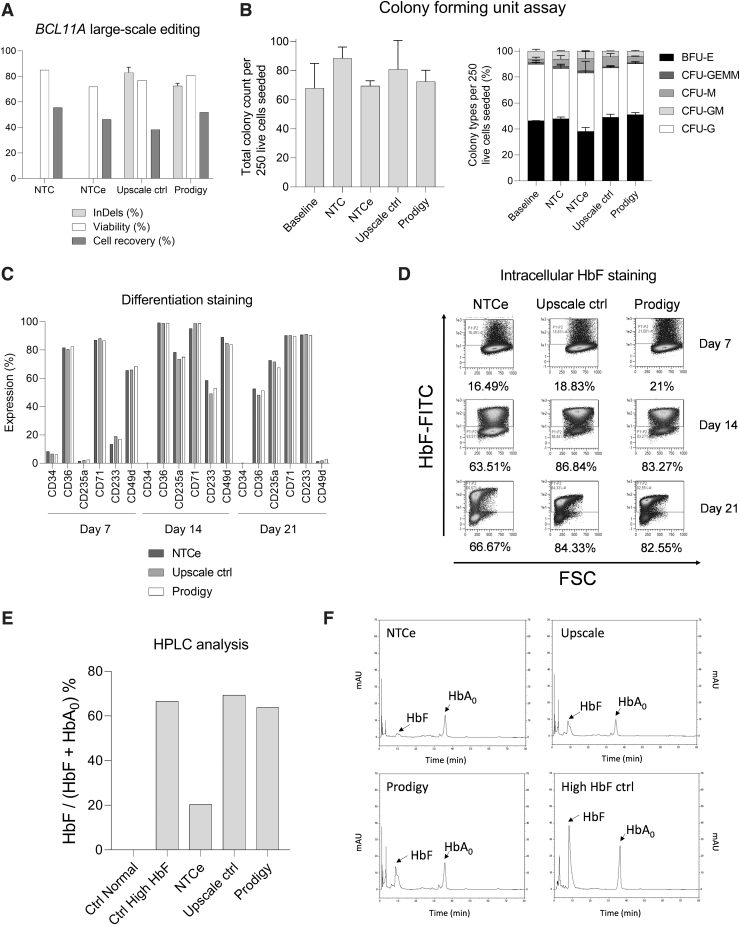
FIG. 2.
Large-scale BCL11A editing of HSPCs using CliniMACS Prodigy system with electroporator compared with NTC, NTCe, and upscale controls using the CliniMACS Prodigy EP-2 cuvette. (A) BCL11A editing at the genomic level (n = 1 with technical replicates), cellular viability, and recovery at day 2 after electroporation (n = 1). (B) CFU assay of large-scale samples compared with upscale samples. Total colonies counted for 250 seeded HSPCs (left) and proportion of different colonies, n = 1 with technical replicates. (C) Erythroid differentiation staining on days 7, 14, and 21. Positive rate by flow cytometry for CD34, CD36, CD235a, CD71, CD233, and CD49d, mock electroporated cells (NTCe) (dark gray), upscale control electroporation (light gray), and cells electroporated by the CliniMACS Prodigy process (white), n = 1. (D) HbF levels of electroporated samples measured by flow cytometry on days 7, 14, and 21. (E) HbF/(HbF+HbA0) ratio as determined by HPLC analysis of normal control cells, HbF expressing control cells, and processed cells after additional erythroid differentiation: mock electroporated cells (NTCe), upscale control electroporated cells, and cells electroporated by the CliniMACS Prodigy process, n = 1. (F) HPLC chromatograms of HbF expressing control cells and electroporated samples after in vitro erythroid differentiation. BFU-E, burst-forming unit-erythroid; CFU, colony-forming unit; CFU-G, CFU-granulocyte; CFU-GEMM, CFU-granulocyte erythrocyte macrophage megakaryocyte; CFU-GM, CFU-granulocyte macrophage; CFU-M, CFU-macrophage; HbF, fetal hemoglobin; HPLC, high-performance liquid chromatography; NTC, nontransfected controls; NTCe, electroporated nontransfected controls.