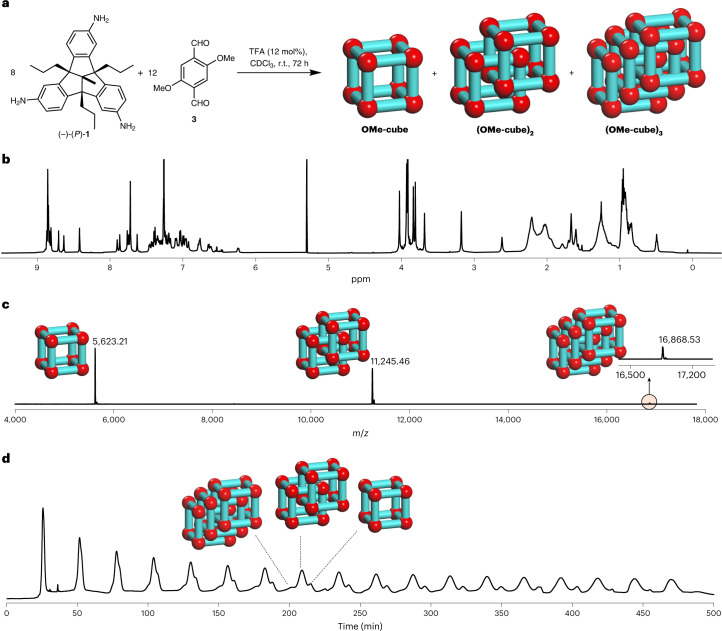
Fig. 2. Synthesis and analysis of monomeric cage and dimeric and trimeric catenanes.
a, Schematic representation of the acid-catalysed 24-fold imine condensation of 1 and 3 to OMe-cubes. b,c, 1H NMR (600 MHz, CDCl3) (b) and MALDI–TOF mass spectrum (DCTB) (c) of the crude reaction mixture of OMe-cubes. The 1H NMR spectrum of the crude product was very complex but that the MALDI–TOF mass spectrum was relatively clear with three distinct peaks. Peaks in the MALDI–TOF mass spectra are labelled with the structures of the products; the small peak for the trimeric catenane is highlighted and shown in the inset. d, r-GPC traces (solvent, DCM) of the crude reaction mixture of OMe-cube, (OMe-cube)2 and (OMe-cube)3 clearly show three peaks.