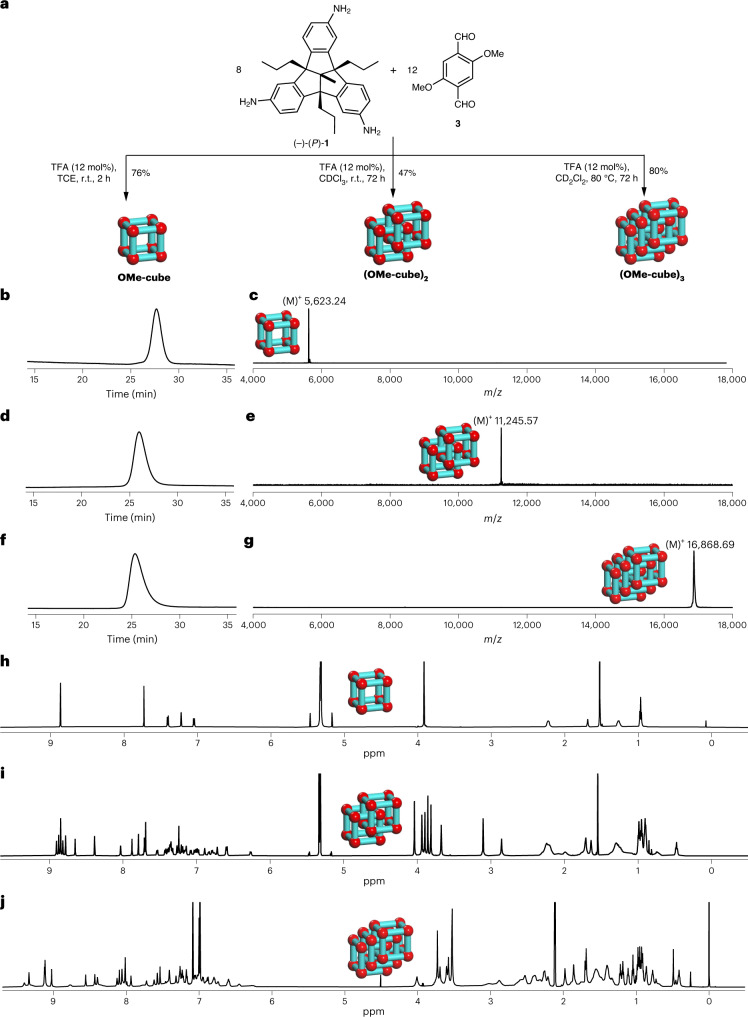
Fig. 3. Selective synthesis and characterization of OMe-cube, (OMe-cube)2 and (OMe-cube)3.
a, Schematic representation of the acid-catalysed 24-fold imine condensation of 1 and 3 in different solvents for the selective formation of OMe-cube, (OMe-cube)2 and (OMe-cube)3. For reaction details, see Supplementary Information, section 2. b–g, r-GPC traces and MALDI–TOF mass spectra of OMe-cube, (OMe-cube)2 and (OMe-cube)3. r-GPC traces (solvent, DCM) of pure OMe-cube (b), (OMe-cube)2 (d) and (OMe-cube)3 (f) show retention times of 27.7, 25.9 and 25.4 min, respectively, which are consistent with their sizes. Depicted is the first cycle for each. The corresponding MALDI–TOF mass spectra of pure OMe-cube (c), (OMe-cube)2 (e) and (OMe-cube)3 (g) show exclusively a single peak for each species. h,i, 1H NMR spectra (600 MHz, 295 K, CD2Cl2) of pure OMe-cube (h) and (OMe-cube)2 (i). j, 1H NMR spectra (700 MHz, 375 K, toluene-d8) of pure (OMe-cube)3.