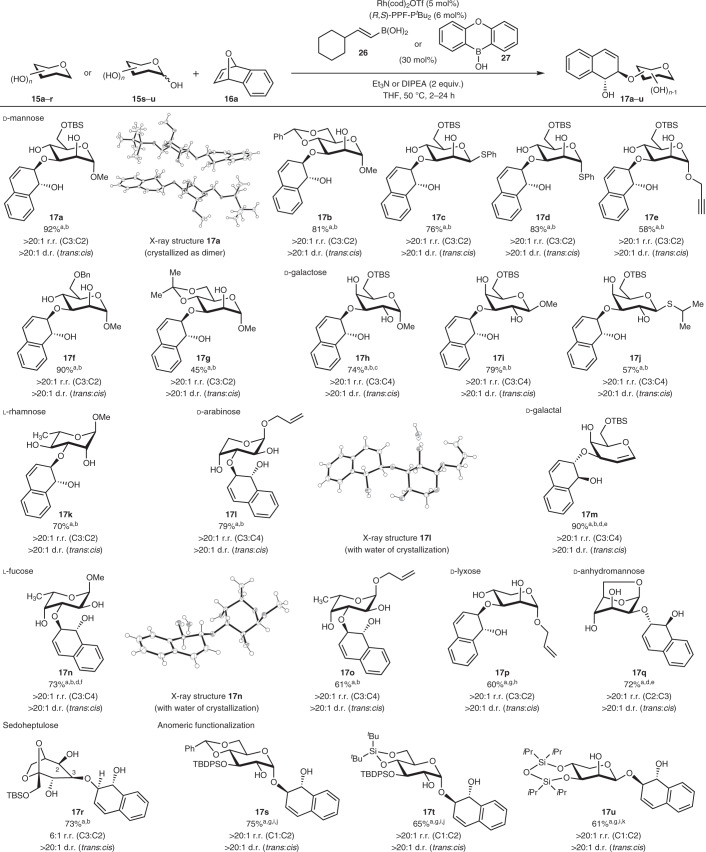
Table 2.
Substrate scope with respect to the carbohydrate polyol
Conditions: [a] Polyol 15a–p (0.2 mmol), 16a (0.4 mmol), Rh(cod)2OTf (5 mol%), (R,S)-PPF-PtBu2 (6 mol%), organoboron catalyst (30 mol%), in THF (2 ml), argon, 50 °C, 24 h. r.r. and d.r. were determined by analysis of the crude 1H NMR spectra. [b] 26 was used as the catalyst. [c] 48 h reaction time. [d] 0.5 ml THF was used. [e] (S,R)-PPF-PtBu2 was used instead. [f] 12 h reaction time. [g] 27 was used. [h] 6% of C3,C2-disubstituted product was isolated (Supplementary Note 14), 1.5 equiv. 16a (0.3 mmol) was used. [i] DIPEA was used as the base instead. [j] 2 h reaction time. [k] 13 h reaction time. Bn, benzyl; cod,1,5-cyclooctadiene; TBS, tert-butyldimethylsilyl; TBDPS, tert-butyldiphenylsilyl. For X-ray structures, thermal ellipsoids shown at 50% probability.