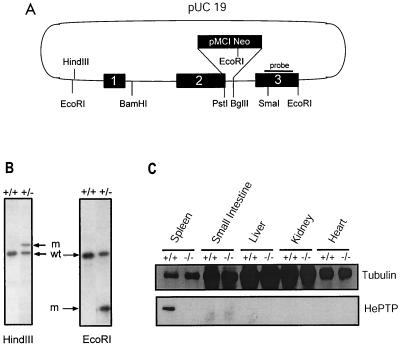
FIG. 1.
Generation of mice deficient in HePTP through homologous recombination. (A) A 2.6-kb EcoRI-EcoRI genomic fragment encompassing the first three exons of HePTP was cloned into pUC19. An antisense neo gene cassette replaced sequence between the PstI site in exon 2 and the BglII site found in the second intron. This construct was transfected into the E14K ES cell line by electroporation. G418-resistant ES clones were screened by PCR using primers at positions indicated by arrows. The solid bar indicates the probe used for Southern confirmation of homologous recombination. (B) Southern analysis of DNA from PCR-positive ES cells, demonstrating successful single-site homologous integration. wt, wild-type ES; m, mutant ES. The mutant HindIII-cut fragment is increased in size by the inserted neomycin gene, while the mutant EcoRI fragment is reduced in size due to the introduction of a new EcoRI site within the neomycin gene. (C) Northern blot analysis of tissues from HePTP wild-type and −/− mice for HePTP mRNA expression. The wild-type form of the mRNA (arrow) is expressed in thymus and spleen cells obtained from wild-type animals but not from −/− mice. In the latter, a higher-molecular-weight form is expressed as a consequence of the neo cassette insertion. A human β-actin probe (Clontech) was used as a loading control. (D) Western blot analysis of tissues from HePTP+/+ and HePTP−/− mice for HePTP expression. While HePTP+/+ mice express this phosphatase in spleen, HePTP−/− mice show no expression in any organ, confirming gene targeting. Equal amounts of total protein (20 μg) were loaded in each lane. Antitubulin immunoblotting demonstrates organ-specific equivalence.