Abstract
Lymphatic metastasis is the leading cause responsible for recurrence and progression in papillary thyroid cancer (PTC), where dysregulation of long non-coding RNAs (lncRNAs) has been extensively demonstrated to be implicated. However, the specific lymphatic node metastatsis-related lncRNAs remain not identified in PTC yet. Lymphatic node metastatsis-related lncRNA, MFSD4A-AS1, was explored in the PTC dataset from The Cancer Genome Atlas and our clinical samples. The roles of MFSD4A-AS1 in lymphatic metastasis were investigated in vitro and in vivo. Bioinformatic analysis, luciferase assay and RNA immunoprecipitation assay were performed to identify the potential targets and the underlying pathway of MFSD4A-AS1 in lymphatic metastasis of PTC. MFSD4A-AS1 was specifically upregulated in PTC tissues with lymphatic metastasis. Upregulating MFSD4A-AS1 promoted mesh formation and migration of human umbilical vein endothelial cells and invasion and migration of PTC cells. Importantly and consistently, MFSD4A-AS1 promoted lymphatic metastasis of PTC cells in vivo by inducing the lymphangiogenic formation and enhancing the invasive capability of PTC cells. Mechanistic dissection further revealed that MFSD4A-AS1 functioned as competing endogenous RNA to sequester miR-30c-2-3p, miR-145-3p and miR-139-5p to disrupt the miRNA-mediated inhibition of vascular endothelial growth factors A and C, and further activated transforming growth factor (TGF)-β signaling by sponging miR-30c-2-3p that targeted TGFBR2 and USP15, both of which synergistically promoted lymphangiogenesis and lymphatic metastasis of PTC. Our results unravel novel dual mechanisms by which MFSD4A-AS1 promotes lymphatic metastasis of PTC, which will facilitate the development of anti-lymphatic metastatic therapeutic strategy in PTC.
Keywords: MFSD4A-AS1, TGF-β signaling, lymph node metastasis, papillary thyroid cancer
Introduction
Thyroid cancer is one of the most commonly seen endocrine malignancies with a clear increased incidence worldwide in recent years (Albores-Saavedra et al. 2007, Blomberg et al. 2012). Thyroid cancer can be divided into papillary thyroid cancer (PTC), follicular thyroid cancer, anaplastic thyroid cancer (ATC) and medullary thyroid cancer based on histological classification, where PTC as the most common histological type accounts for approximately 90% of all thyroid cancer types (Mao & Xing 2016). Although the vast majority of PTC patients have an excellent prognosis with 5-year survival rate exceeding 95% after treated by surgical removal, followed by adjuvant radioactive iodine therapy (Hay et al. 2002), lymph node metastasis of PTC is the principal clinical issue affecting the prognosis of PTC patients (Zhang et al. 2019). Therefore, the identification of lymph node metastasis-relevant factors will facilitate early detection of lymph node metastasis and the development of anti-lymph node metastasis therapeutic strategy in PTC patients.
Lymph node metastasis is a dynamic and sequential biological process arising from primary tumors (Stacker et al. 2014). Once tumor cells metastasize to and colonized in the lymph node, tumor-secreted cytokines, such as vascular endothelial growth factors C (VEGFC) and VEGFD, induce proliferation and growth of new lymphatic vessels (lymphangiogenesis) by binding to VEGF receptors on lymphatic endothelial cells, which further promotes the tumor cells metastasizing to distant lymph nodes in the next echelon in an orderly sequence through lymphaticovenous connections to the general systemic circulation (Nathanson 2003). Lymphangiogenesis is characterized by tumor cell-induced proliferation and migration of lymphatic endothelial cells and the formation of new lymphatic capillaries in which VEGFs are reported to play the most important role (Joukov et al. 1996). Of note, several lines of evidence have shown that VEGFC plays a critical role in lymphangiogenesis of PTC, and overexpression of VEGFC predicted higher lymphatic metastasis risk in PTC patients (Yu et al. 2005, Yu et al. 2008). Importantly, inhibition of VEGFs signaling can suppress tumor lymphangiogenesis and metastasis to regional and distant lymph nodes in a variety of cancer types (He et al. 2002, Nakamura et al. 2004, Roberts et al. 2006, Burton et al. 2008). Hence, further investigation of the VEGFs regulator might provide a potential therapeutic strategy for PTC patients with lymph node metastasis.
The long non-coding RNAs (lncRNAs) are a class of ncRNA with lengths of more than 200 nucleotides and are implicated in multiple biological processes, including as enhancers to regulate the target genes transcription as scaffold molecules to modulate proteins/genes or proteins/proteins interactions (Ponting et al. 2009, Salehi et al. 2017). Furthermore, a great deal of attention has focused on the role of lncRNAs as competing endogenous RNAs (ceRNA) to sequester target miRNAs to disrupt the miRNAs-mediated degradation of target genes (Karreth et al. 2011), and dysregulation of miRNAs has been well established to promote tumor tumorigenesis, progression and metastasis in numerous cancer types (Zhang et al. 2018, Ren et al. 2017a, b, Dai et al. 2019, Peng et al. 2019). Furthermore, the pivotal role of lncRNAs in the development, progression and metastasis of various types of cancer has seized great momentum in accumulating studies (Lang et al. 2020, Liu et al. 2020, Chen et al. 2020c, Lang et al. 2021), including lymph node metastasis (Chen et al. 2018, Chen et al. 2020a). Notably, several independent studies have shown that lncRNAs may hold clinical applicable value as the potential predictive markers for early detection of lymph node metastasis in PTC (Feng et al. 2019, Zhuang et al. 2019, Li et al. 2020). However, the functional role of lncRNAs in lymphatic metastasis of PTC in vivo, as well as the underlying mechanism is still largely unknown. Therefore, an in-depth dissection of the functional role of lncRNAs and clarification of the underlying mechanism in lymphatic metastasis of PTC will deepen our understanding about the clinical and biological significance of lncRNAs in lymphatic metastasis of PTC.
In the current study, our findings combined with the analytic results of PTC dataset from The Cancer Genome Atlas (TCGA) revealed that lncRNA MFSD4A-AS1 was specifically upregulated in PTC tissues with lymph node metastasis. Gain- and loss-of-function assays showed that upregulating MFSD4A-AS1 promoted, while silencing MFSD4A-AS1 suppressed lymphatic metastasis of PTC cells in vivo, and proliferation and migration of human umbilical vein endothelial cells (HUVECs) and invasion and migration of PTC cells in vitro. Mechanistic investigations further demonstrated that upregulating MFSD4A-AS1 promoted lymphatic metastasis of PTC by sequestering miR-30c-2-3p, miR-145-3p and miR-139-5p to disrupt the miRNAs-mediated inhibition of VEGFA and VEGFC and inactivation of transforming growth factor (TGF)-β signaling. Collectively, our results provide experimental evidence regarding the clinical significance and biological role of MFSD4A-AS1 in lymphatic metastasis of PTC, which will facilitate early detection of lymph node metastasis and the development of anti-lymph node metastasis therapeutic methods in PTC patients.
Materials and methods
Cell lines and cell culture
Normal primary thyroid follicular epithelial (PTFE) cells were purchased from Procell (Procell Life Science & Technology Co., Ltd., Wuhan, China). Thyroid cancer cell lines, including PTC cell lines (B-CPAP, K1 and KTC-1) and ATC cell lines (BHT-101, CAL-62, KMH-2 and 8305C), were obtained from Cell Bank of Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). PTFE cells were cultured in CM-H023 medium (Procell), and thyroid cancer cell lines were cultured in RPMI-1640 medium (Life Technologies) supplemented with penicillin G (100 U/mL), streptomycin (100 mg/mL) and 10% fetal bovine serum (FBS, Life Technologies). All cell lines were cultured at 37°C in a humidified atmosphere with 5% CO2.
Patients and tumor tissues
A total of 48 paired PTC tissues and the matched adjacent normal tissues, and 54 separate PTC tissues were obtained during surgery at the Division of Thyroid Surgery, China-Japan Union Hospital of Jilin University, between January 2016 and December 2020 (Supplementary Table 1, see section on supplementary materials given at the end of this article). Patients were diagnosed based on clinical and pathological evidence, and the specimens were immediately snap-frozen and stored in liquid nitrogen tanks. For the use of these clinical materials for research purposes, prior patients’ consents and approval from the Institutional Research Ethics Committee of Jilin University were obtained.
Plasmid and transfection
Human MFSD4A-AS1 cDNA (Vigene Biosciences, Shandong, China) was cloned into the pcDNA3.1(+) or GV350 plasmid (GenChem, Shanghai, China). Knockdown of endogenous MFSD4A-AS1 was performed by cloning two short hairpin RNA (shRNA) oligonucleotides into the GV493 vector (GenChem). The sequences of the two separate shRNA fragments are listed in Supplementary Table 2. The 3′UTR regions of VEGFA and VEGFC and the region of MFSD4A-AS1 sequences targeted by miR-30c-2-3p, miR-145-3p or miR-139-5p were PCR-amplified from genomic DNA and cloned into pmirGLO vectors (Promega), and the list of primers used in cloning reactions was provided in Supplementary Table 2. miR-30c-2-3p, miR-145-3p and miR-139-5p agomirs was synthesized and purified by RiboBio. The (CAGAC) 12/pGL3 TGF-β/Smadresponsive luciferase reporter plasmid and the control plasmids (Clontech, Kusatsu, Shiga, Japan) were used to quantitatively assess the transcriptional activity of TGF-β signaling components. Transfection of plasmids was performed as previously described (Wu et al. 2018).
RNA extraction, reverse transcription and real-time PCR
RNA from tissues and cells was extracted (TRIzol, Life Technologies) according to the manufacturer’s instructions. Messenger RNA (mRNA), lncRNA and miRNA were reverse transcribed from the total RNA using the Revert Aid First Strand cDNA Synthesis Kit (Thermo, Carlsbad, California, USA) according to the manufacturer’s protocol. Complementary DNA was amplified and quantified on the ABI 7500HT system (Applied Biosystems) using SYBR Green I (Applied Biosystems). The primers used in the reactions are listed in Supplementary Table 3. Real-time PCR was performed as described previously (Miller et al. 2015). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as endogenous control for mRNA and lncRNA. Relative fold expressions were calculated with the comparative threshold cycle (Dai et al. 2017).
Western blotting analysis
Western blot was performed according to a standard method, as described previously (Zhang et al. 2017b). Antibodies against TGFBR2 (mouse mAb, Cat#: 66636-1-Ig, 1:2000), USP15 (Mouse mAb, Cat#: 67557-1-Ig, 1:5000) and phosphorylated SMAD3 (Rabbit mAb, Cat#: ab52903, 1:5000) were purchased from Proteintech and Abcam. The membranes were stripped and reprobed with an α-tubulin (mouse mAb, Cat#: ab7291, 1:5000) and p84 (Rabbit mAb, Cat#: ab131268, 1:2000) antibody as the loading control for cytoplasm and nucleus.
Invasion and migration assays
The invasion and migration assays were performed as described previously (Ren et al. 2013). The invasion assay was performed by using Transwell chamber consisting of 8‑mm membrane filter inserts (Corning) coated with Matrigel (BD Biosciences). 1.5 × 105 K1 and B-CPAP cells were added to the upper chamber, and the lower chamber was filled with the medium with 10% FBS. The experiments were repeated three times, and the cell count was performed under a microscope (×100).
Cell counting kit-8 analysis
2 × 103 K1 and B-CPAP cells were seeded into 96-well plates, and the specific staining process and methods were performed according to the previous study (Zhang et al. 2017a ). The experiments were repeated three times.
Colony formation assay
The K1 and B-CPAP cells were trypsinized as single cell and suspended in the media with 10% FBS. The indicated cells (300 cells per well) were seeded into of 6-well plate for ~10–14 days. Colonies were stained with 1% crystal violet for 10 min after fixation with 10% formaldehyde for 5 min. Plating efficiency was calculated as previously described (Wang et al. 2016). Different colony morphologies were captured under a light microscope (Olympus). The experiments were repeated three times.
Tube formation of human umbilical vein endothelial cells
Precooled Matrigel (Corning) was added into wells of a 24-well plate and polymerized for 30 min at 37°C. Human umbilical vein endothelial cells (HUVECs) (2 × 104) suspended in 200 μL of conditional medium were added to each well and incubated with the supernatants from the indicated K1 and B-CPAP cells at 37°C in 5% CO2 for 6–12 h. The images of tube structure were captured under a 100× bright-field microscope, and quantification of tube formation was measured by the mesh and length of the completed tubes by Image View 3.7 (Jingtong, China). The experiments were repeated three times.
Migration assay
4 × 104 K1 and B-CPAP cells were seeded into the upper compartment of the 24-well Transwell permeable chambers (Corning), and the lower chamber of the Transwell was filled with completed media supplemented with 10% FBS as a chemoattractant. After incubation for 24 h, cells migrated to the bottom side of the chamber were fixed with stationary solution (methanol:acetic acid = 3:1), stained with crystal violet and photographed and quantified by counting in five random fields. The experiments were repeated three times.
RNA immunoprecipitation
Cells were co-transfected with hemagglutinin (HA)–Ago2, followed by HA–Ago2 immunoprecipitation using HA antibody. Real-time PCR analysis of the IP material was used to test the association of the mRNA of VEGFA and VEGFC with the RNA-induced silencing complex. The detailed processes were performed as described previously (Li et al. 2017).
Animal study
Eight-week-old BALB/c-nu mice were purchased from the Experimental Animal Center of the Guangzhou University of Chinese Medicine, and their care was in accordance with institution guidelines. The mice were randomly divided into three groups (n = 6 per group) and the indicated K1 cells (1 × 106) were injected into the footpad of the mice, or subcutaneously. The primary tumors were allowed to form, then the mice were euthanized on the end-points, and the inguinal lymph nodes were excised and paraffin-embedded. Sections of the lymph nodes were subjected to H & E staining, and CD31, PDPN and Ki-67 staining for histological examination, and the tumor cell number was counted as previously described (Chen et al. 2020b). The ethic approval of animal study was obtained from The Tab of Animal Experimental Ethical Inspection of Jilin University.
Luciferase assay
The K1 and B-CPAP cells (4 × 104) were seeded in triplicate in 24-well plates and cultured for 24 h, and the luciferase reporter assay was performed as previously described (Ren et al. 2019). Cells were transfected with 250 ng (CAGAC) 12/pGL3 reporter luciferase plasmid or 5 ng pRL-TK Renilla plasmid (Promega) using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s recommendation. Luciferase and Renilla signals were measured 36 h after transfection using a Dual Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s protocol.
Enzyme-linked immunosorbent assay
Cells (5 ×105) were seeded into six-well plates and incubated for 24 h at 37°C in 5% CO2 atmosphere. Then, the conditional media (without FBS) was collected and used in order to determine the secretion of VEGFA and VEGFC, using the VEGF ELISA kit (Raybiotech, Guangzhou, Guangdong, China) in accordance with the manufacturer's protocol. Absorbance was measured on a Synergy 2 microplate system (BioTek) at 450 nm.
Statistical analysis
All values are presented as means ± s.d.. Significant differences were determined using GraphPad 5.0 software. Student’s t-test was used to determine statistical differences between two groups when the groups are not matched, and a paired analysis is used to determine statistical differences between two groups when the groups are matched. One-way ANOVA was used to determine statistical differences between multiple testing. P < 0.05 was considered significant. All the experiments were repeated three times.
Results
lncRNA-MFSD4A-AS1 is specifically upregulated in PTC tissues with lymph node metastasis
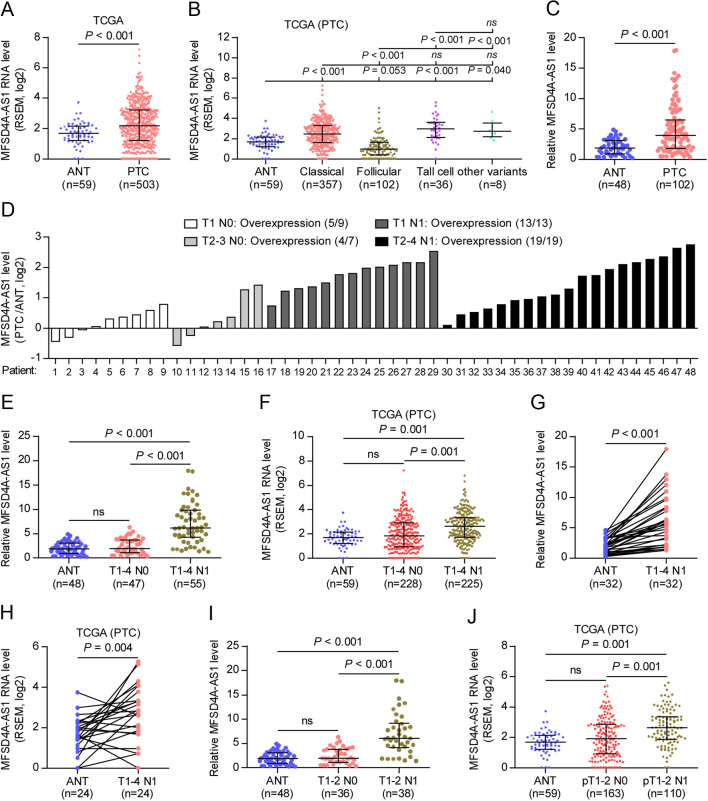
To explore the lymph node metastasis-associated lncRNAs, we first investigated the RNA sequencing dataset of PTC from TCGA. As shown in Fig. 1A and Supplementary Fig. 1A, we found that lncRNA-MFSD4A-AS1 expression was increased in separate 503 PTC tissues, 59 paired PTC tissues compared with the adjacent normal tissues (ANT). In addition, 503 PTC was further sub-categorized into 357 classic PTC, 102 follicular variants of PTC, 36 tall cell variants of PTC and 8 other variants, including 4 diffuse sclerosing variants, 2 cribriform morular variants, 1 columnar cell variant and 1 papillary and follicular variant, and MFSD4A-AS1 level was differentially increased in classic, tall cell and other variants, but decreased in follicular variant compared with that in ANT (Fig. 1B). Consistently, MFSD4A-AS1 expression was demonstrated to be higher in our PTC without distant metastasis tissues than that in ANT (Fig. 1C). To further underscore the clinical significance of MFSD4A-AS1 in PTC with lymph node metastasis (TC-N1), we stratified PTC tissues based on lymph node metastatic status. First, we found that MFSD4A-AS1 was elevated in all TC-N1 tissues compared to PTC tissues without lymph node metastasis (TC-N0) by analyzing our 48 paired PTC tissues (Fig. 1D). Of note, MFSD4A-AS1 expression was specifically and significantly upregulated in TC-N1 tissues compared with that in ANT in our and TCGA datasets (Fig. 1E and F), but there was no statistical difference of MFSD4A-AS1 expression between TC-N0 and ANT (Fig. 1E and F), including in T1–2 and T3–4 subgroups, respectively (Supplementary Fig. 1B and C). Importantly, MFSD4A-AS1 was also specifically elevated in paired TC-N1 tissues compared with the matched ANT in our and TCGA datasets (Fig. 1G and H), and there was no statistical difference between paired TC-N0 tissues and ANT (Supplementary Fig. 1D and E). Even in the subgroup of T1–2 PTC, MFSD4A-AS1 was only upregulated in TC-N1 tissues (Fig. 1I and J) but did not show significant differential expression between TC-N0 and ANT (Fig. 1H and I). Collectively, our clinical findings in combination with the results of the PTC dataset from TCGA indicate that MFSD4A-AS1 may play a pivotal role in the lymphatic metastasis of PTC.
Figure 1.
MFSD4A-AS1 is upregulated in PTC with lymph node metastasis. (A) MFSD4A-AS1 expression in 59 adjacent normal tissues (ANT) and 503 PTC tissues in the thyroid cancer dataset from TCGA. (B) MFSD4A-AS1 expression in 357 classic PTC, 102 follicular variants of PTC, 36 tall cell variants of PTC and 8 other variants, including 4 diffuse sclerosing variants, 2 cribriform morular variants, 1 columnar cell variant and 1 papillary and follicular variants, in the thyroid cancer dataset from TCGA. (C) Real-time PCR analysis of MFSD4A-AS1 expression in our 48 ANT and 102 PTC tissues without distant metastasis. GAPDH was used as endogenous control. (D) Real-time PCR analysis of MFSD4A-AS1 expression relative to their match ANT in our 48 paired PTC tissues, including 9 T1 PTC tissues without lymphatic metastasis, 7 T2–3 PTC tissues without lymphatic metastasis, 13 T1 PTC tissues with lymphatic metastasis and 19 T2–4 PTC tissues with lymphatic metastasis. GAPDH was used as endogenous control. (E) Real-time PCR analysis of MFSD4A-AS1 expression in our 48 ANT, 47 T1–4 PTC tissues without lymphatic metastasis and 65 T1–4 PTC tissues with lymphatic metastasis. GAPDH was used as endogenous control. n.s. indicates no significant difference. (F) MFSD4A-AS1 expression in 59 ANT, 230 T1–4 PTC tissues without lymphatic metastasis and 225 T1–4 PTC tissues with lymphatic metastasis in the thyroid cancer dataset from TCGA. n.s. indicates no significant difference. (G) Real-time PCR analysis of MFSD4A-AS1 expression in our 32 paired PTC tissues with lymphatic metastasis and the matched adjacent normal tissues. (H) MFSD4A-AS1 expression in 24 paired PTC tissues with lymphatic metastasis and the matched adjacent normal tissues in the PTC dataset from TCGA. (I) Real-time PCR analysis of MFSD4A-AS1 expression in our 48 ANT, 36 T1–2 PTC tissues without lymphatic metastasis and 38 T1–2 PTC tissues with lymphatic metastasis. GAPDH was used as endogenous control. n.s. indicates no significant difference. (J) MFSD4A-AS1 expression in 59 ANT, 164 T1-2 PTC tissues without lymphatic metastasis and 110 T1-2 PTC tissues with lymphatic metastasis in the PTC dataset from TCGA. n.s. indicates no significant difference.
MFSD4A-AS1 promotes lymphatic metastasis by promoting lymphangiogenesis-inducing and invasive abilities of PTC cells in vivo
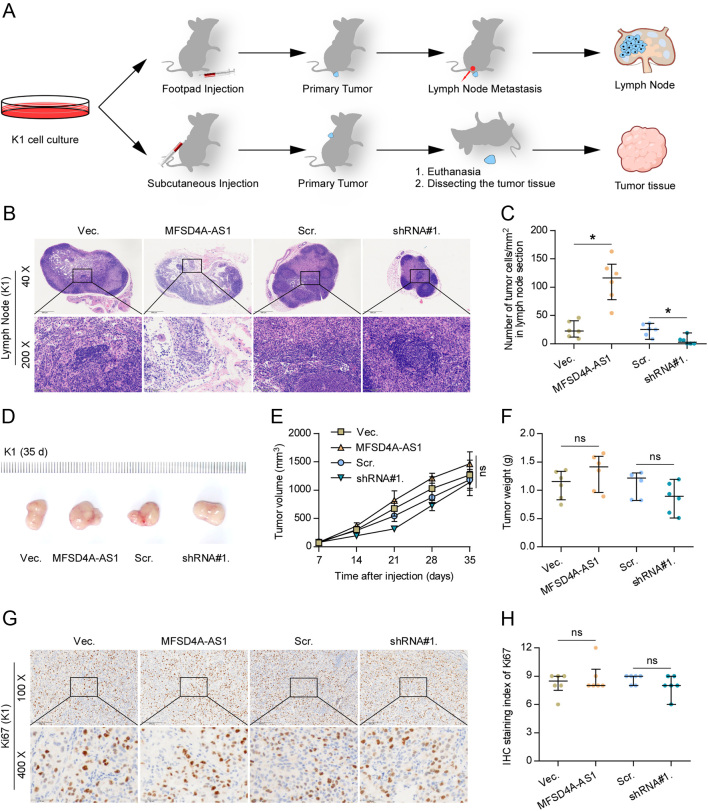
To determine the role of MFSD4A-AS1 in lymphatic metastasis of PTC cells, we first examined the expression levels of MFSD4A-AS1 in a normal thyroid follicular epithelial cell line PTFE and seven thyroid cancer cell lines and found that MFSD4A-AS1 expression levels were higher to a different extent in thyroid cancer cells than that in PTFE cells (Supplementary Fig. 2A). We further endogenously knocked down MFSD4A-AS1 expression and stably overexpressed MFSD4A-AS1 in B-CPAP and K1 cells, because these two cell lines showed moderate levels of MFSD4A-AS1 compared to other thyroid cancer cell lines (Supplementary Fig. 2B), and the tumorigenic role of K1 in animal experiment in vivo was markedly superior to other PTC cell lines based on our previous experiments. Through the inguinal lymph node metastatic model, we first injected the indicated PTC cells into the surrounding foot pads tissues of the mice (Fig. 2A). Four weeks after cell inoculations, we found that overexpressing MFSD4A-AS1 dramatically increased the number of tumor cells in lymph nodes from the MFSD4A-AS1-overexpressing mice group compared with that in vector mice group (Fig. 2B and C). By contrast, silencing MFSD4A-AS1 reduced the number of tumor cells in lymph nodes derived from the MFSD4A-AS1-silencing mice group (Fig. 2B and C).
Figure 2.
MFSD4A-AS1 promotes lymphatic metastasis of PTC cells in vivo. (A) Schematic model of lymphatic metastasis (upper panel) and s.c. injection (upper panel) model in vivo. (B) H & E staining analysis of tumors in lymph nodes from the indicated mice group. (C) The number of tumor cells in the tumor areas of lymph nodes from the indicated mice group. *P < 0.05. (D) The representative tumors formed by the indicated K1 cells in each mice group (n = 6, each group). (E) The effect of MFSD4A-AS1 on the tumor volumes in the indicated mice groups from the seventh day at 7 days interval after inoculation of 5 × 106 cells. Data presented are the mean ± s.d. n.s. indicates no significant difference. (F) The effect of MFSD4A-AS1 on the tumor weights in the indicated mice groups after inoculation of 5 × 106 cells. n.s. indicates no significant difference. (G) The representative images of Ki-67 staining in the tumor tissues from the indicated mice groups. (H) The Ki-67 staining scores in the tumor tissues from the indicated mice groups. n.s. indicates no significant difference.
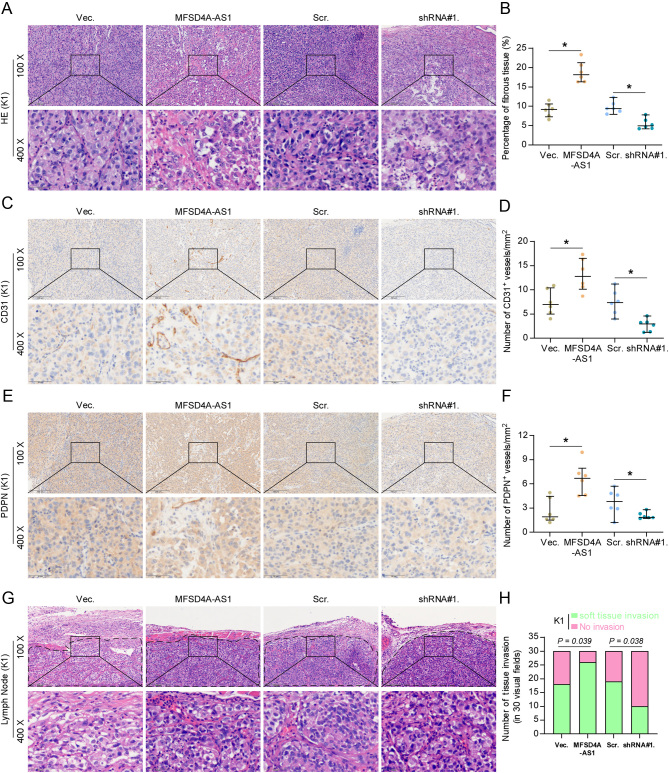
To further investigate the biological function of MFSD4A-AS1 in lymphangiogenesis in vivo, the vector, MFSD4A-AS1-overexpressing, scramble and MFSD4A-AS1-silencing K1 cells were subcutaneously injected into the inguinal folds of the mice (Fig. 2A). As shown in Fig. 2D, E, F, G and H, neither tumor volume or weight nor Ki-67 staining index was affected by changed expression of MFSD4A-AS1 in vivo. Importantly, our results further revealed that upregulating MFSD4A-AS1 increased, while silencing MFSD4A-AS1 reduced the density of lymphatic or blood vessel (LBV) in tumor tissues, as demonstrated by the CD31+ and PDPN+ LBV per mm2 in tumor sections (Fig. 3A, B, C, D, E and F). Microscopic examination of tumor morphology showed that MFSD4A-AS1-overexpressing tumor tissues displayed more potent capabilities to penetrate into the surrounding muscle tissues; conversely, silencing MFSD4A-AS1 drastically inhibited the local invasive capability of tumors (Fig. 3G and H), suggesting the stimulatory effect of MFSD4A-AS1 on the invasive ability of PTC cells in vivo. Taken together, our results demonstrate that MFSD4A-AS1 promotes lymphatic metastasis of PTC cells in vivo by inducing the lymphangiogenic formation and enhancing the invasive capability of PTC cells.
Figure 3.
MFSD4A-AS1 promotes lymphangiogenesis and local invasion of PTC cells in vivo. (A and B) H & E staining analysis, and CD31 and PDPN staining in the tumor tissues from the indicated mice groups. *P < 0.05. (C and D) The CD31 staining scores in the tumor tissues from the indicated mice groups. *P < 0.05. (E and F) The PDPN staining scores in the tumor tissues from the indicated mice groups. *P < 0.05. (G) H & E staining analysis of the local invasive ability of PTC cells in the tumor tissues from the indicated mice groups. (H) The effect of MFSD4A-AS1 on muscle and fat infiltration of the adjacent tissues in the tumor tissues from the indicated mice groups. *P < 0.05.
MFSD4A-AS1 enhances lymphangiogenesis of vascular endothelial cells and invasion and migration of PTC cells
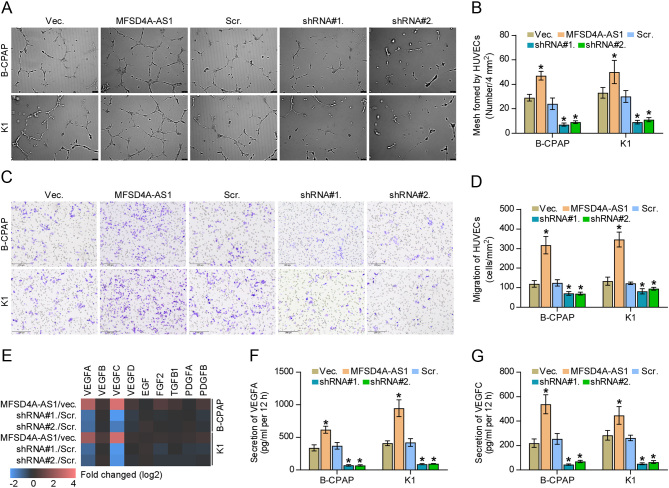
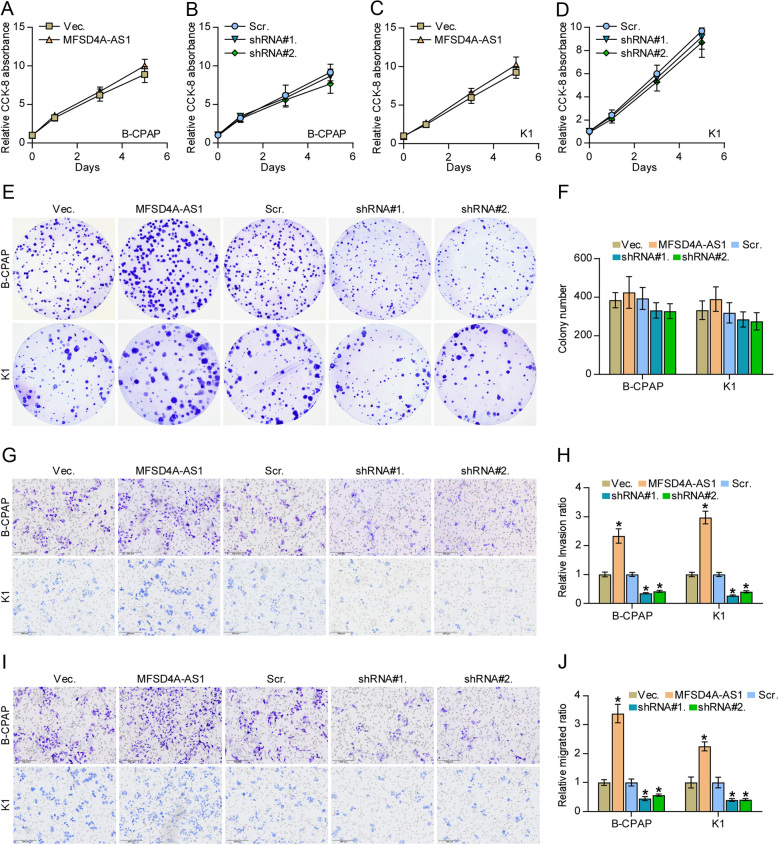
The biological function of MFSD4A-AS1 in lymphatic metastasis of PTC was further determined in a series of functional experiments in vitro. First, we performed tube formation and Transwell assays to investigate the effect of MFSD4A-AS1 on the proliferation and migration of vascular endothelial cells and found that the condition medium from MFSD4A-AS1-overexpressing PTC cells promoted mesh formation by HUVECs and the migration ability of HUVECs, whereas silencing MFSD4A-AS1 exhibited an opposite effect (Fig. 4A, B, C and D). In addition, upregulating MFSD4A-AS1 increased, while silencing MFSD4A-AS1 decreased expression of VEGFA and VEGFC (Fig. 4E). Notably, the secretion levels of both VEGFA and VEGFC were affected by the changed expression of MFSD4A-AS1 (Fig. 4F and G). Consistent with the findings in vivo (Fig. 2D, E, F, G and H), the changed expression of MFSD4A-AS1 had no significant effect on the cell growth of PTC cells (Fig. 5A, B, C, D, E and F). In addition, upregulating MFSD4A-AS1 enhanced, while silencing MFSD4A-AS1 inhibited the invasion and migration capabilities of PTC cells (Fig. 5G, H, I and J). Therefore, our results reveal that MFSD4A-AS1 promotes lymphangiogenesis-inducing, invasive and migration capabilities of PTC cells in vitro.
Figure 4.
MFSD4A-AS1 enhances lymphangiogenesis of vascular endothelial cells of PTC cells. (A and B) The effect of changed expression of MFSD4A-AS1 in PTC cells on human umbilical vein endothelial cells (HUVECs) in tube formation assay. Each bar represents the mean values ± s.d. of three independent experiments. *P < 0.05. (C and D) The effect of changed expression of MFSD4A-AS1 in PTC cells on migration ability of HUVECs in Transwell assay. Each bar represents the mean values ± s.d. of three independent experiments. *P < 0.05. (E) Real-time PCR analysis of the effect of MFSD4A-AS1 on the expression of multiple proliferation and migration of vascular endothelial cells-associated (angiogenesis-associated) genes. Pseudo-color scale values were log2 transformed. Transcript levels were normalized by GAPDH expression. The experiment was independently performed three times. (F and G) The effect of MFSD4A-AS1 on secretion level of VEGFA and VEGFC in PTC cells by enzyme-linked immunosorbent assay (ELISA). Each bar represents the mean values ± s.d. of three independent experiments. *P < 0.05.
Figure 5.
MFSD4A-AS1 promotes invasion and migration abilities but does not affect proliferation in PTC cells. (A–D) The effect of overexpression or silencing MFSD4A-AS1 on the cell growth in the indicated PTC cells by CCK-8 assay. (E and F) The effect of overexpression or silencing MFSD4A-AS1 on colony-formation ability of the indicated PTC cells by colony-formation assay. (G and H) The effect of overexpression or silencing MFSD4A-AS1 on invasion ability of PTC cells. Each bar represents the mean values ± s.d. of three independent experiments. *P < 0.05. (I and J) The effect of overexpression or silencing MFSD4A-AS1 on migration ability of PTC cells. Each bar represents the mean values ± s.d. of three independent experiments. *P < 0.05.
MFSD4A-AS1 functions as a competing endogenous RNA by sponging miR-30c-2-3p, miR-145-3p and miR-139-5p
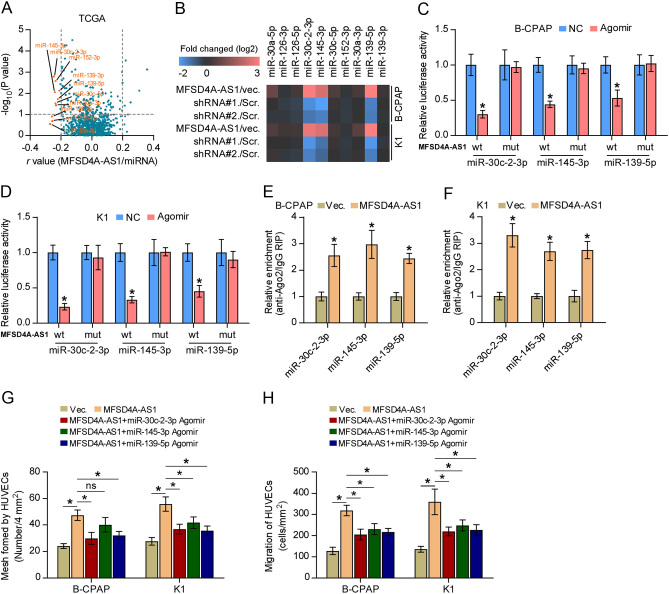
Numerous studies have shown that lncRNAs can serve as competitive endogenous RNAs (ceRNAs) to regulate downstream mRNAs expression by sponging miRNAs (Karreth et al. 2011, Tay et al. 2011). Then, the miRNAs binding to MFSD4A-AS1 were further explored by analyzing the correlation of MFSD4A-AS1 with all reported miRNAs in the PTC dataset from TCGA. As shown in Fig. 6A, the expression levels of ten miRNAs, including miR-30a-5p, miR-126-3p, miR-126-5p, miR-30c-2-3p, miR-145-3p, miR-30c-5p, miR-152-3p, miR-30a-3p, miR-139-5p and miR-139-3p, were significantly inversely correlated with MFSD4A-AS1 expression. Real-time PCR analysis further demonstrated that only miR-30c-2-3p, miR-145-3p and miR-139-5p were significantly affected by the changed expression of MFSD4A-AS1 (Fig. 6B). Notably, miR-139-5p was reported to serve as a prognostic marker in PTC patients and functioned as a tumor-suppressive miRNA in PTC (Montero-Conde et al. 2020). Importantly, miR-30c-2-3p was found to be positively correlated with lymph node metastasis of PTC (Saiselet et al. 2015). Through analyzing publicly available algorithms, we found that miR-30c-2-3p, miR-145-3p and miR-139-5p had the potential recognition sequences on MFSD4A-AS1 (Supplementary Fig. 3A, B and C). Luciferase assay demonstrated that miR-30c-2-3p, miR-145-3p and miR-139-5p agomir reduced the 3'UTR reporter activity of MFSD4A-AS1, but not of the mutant 3'UTR of MFSD4A-AS1 (Fig. 6C and D). RNA immunoprecipitation (RIP) assays demonstrated direct associations of miR-30c-2-3p, miR-145-3p and miR-139-5p with the transcripts of MFSD4A-AS1 (Fig. 6E and F). Importantly, upregulating miR-30c-2-3p, miR-145-3p and miR-139-5p differentially attenuated the pro-mesh formation and migration ability of MFSD4A-AS1 overexpression in PTC cells (Fig. 6G and H). Thus, these results indicate that MFSD4A-AS1 sponge miR-30c-2-3p, miR-145-3p and miR-139-5p as a ceRNA to inhibit their expression.
Figure 6.
MFSD4A-AS1 sponges miR-30c-2-3p, miR-145-3p and miR-139-5p. (A) Volcano plot analyzed the clinical correlation of MFSD4A-AS1 with all reported miRNAs in the PTC dataset from TCGA. The orange colors represent significantly and negatively correlated miRNAs with fold change > 2 and r value < −0.2. (B) Real-time PCR analysis of the effect of MFSD4A-AS1 on expression of miR-30a-5p, miR-126-3p, miR-126-5p, miR-30c-2-3p, miR-145-3p, miR-30c-5p, miR-152-3p, miR-30a-3p, miR-139-5p and miR-139-3p. Pseudo-color scale values were log2 transformed. Transcript levels were normalized by U6 expression. The experiment was independently performed three times. (C and D) The effect of miR-30c-2-3p, miR-145-3p and miR-139-5p on the luciferase activity of wild-type or mutant MFSD4A-AS1 in the indicated cells. Error bars represent the mean ± s.d. of three independent experiments. *P < 0.05. (E and F) RNA immunoprecipitation (RIP) assay showing the association among miR-30c-2-3p, miR-145-3p and miR-139-5p, and MFSD4A-AS1 transcripts in PTC cells. Pulldown of IgG antibody served as the negative control *P < 0.05. (G) The mediating effect of changed expression of miR-139-5p, miR-145-3p and miR-30c-2-3p in PTC cells on human umbilical vein endothelial cells (HUVECs) in tube formation assay. Each bar represents the mean values ± s.d. of three independent experiments. *P < 0.05. (H) The mediating effect of changed expression of miR-139-5p, miR-145-3p and miR-30c-2-3p in PTC cells on migration ability of HUVECs in the Transwell assay. Each bar represents the mean values ± s.d. of three independent experiments. *P < 0.05.
MFSD4A-AS1 elevates VEGFA and VEGFC dependent on different downstream miRNAs
By analyzing multiple publically available algorithms, we found that VEGFC may be the potential targets of miR-30c-2-3p and miR-145-3p, and VEGFA be the target of miR-139-5p (Supplementary Fig. 4A). Real-time PCR analysis demonstrated that upregulating miR-30c-2-3p and miR-145-3p differentially reduced the mRNA levels of VEGFC, and only overexpression of miR-139-5p decreased VEGFA expression in PTC cells (Supplementary Fig. 4B and C); conversely, silencing miR-30c-2-3p and miR-145-3p elevated VEGFC expression, and downregulating miR-139-5p enhanced VEGFA expression (Supplementary Fig. 4B and C). Consistently, ELISA assay further showed that upregulating miR-30c-2-3p and miR-145-3p decreased while silencing miR-30c-2-3p and miR-145-3p increased the secretion levels of VEGFC; upregulating miR-139-5p reduced while silencing miR-139-5p increased the secretion levels of VEGFA (Supplementary Fig. 4D and E). RIP assay demonstrated direct correlations of miR-30c-2-3p and miR-145-3p with the transcript of VEGFC and miR-139-5p with VEGFA transcript (Supplementary Fig. 4F and G). Luciferase assay further revealed that upregulating miR-30c-2-3p and miR-145-3p decreased, whereas silencing miR-30c-2-3p and miR-145-3p enhanced the 3'UTR reporter activity of VEGFC, and changed expression of miR-139-5p had negative effect on the 3'UTR reporter activity of VEGFA, but not of the mutant ones (Supplementary Fig. 4H and I). Further investigation showed that only miR-139-5p reduced VEGFA secretion in MFSD4A-AS1-overexpressing PTC cells (Supplementary Fig. 4J). However, miR-30c-2-3p and miR-145-3p but not miR-139-5p could reverse the stimulatory effect of MFSD4A-AS1 overexpression on secretion levels of VEGFC (Supplementary Fig. 4K). Therefore, these findings demonstrate that MFSD4A-AS1 enhances VEGFA and VEGFC expression and promotes their secretion by sponging miR-139-5p, and miR-30c-2-3p and miR-145-3p, respectively, to disrupt the inhibitory effects of these three miRNAs on VEGFA and VEGFC.
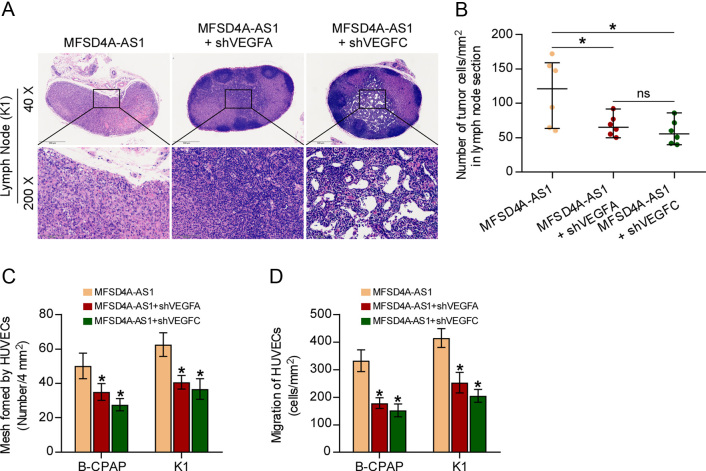
MFSD4A-AS1 promotes lymphatic metastasis and proliferation and migration of HUVECs by upregulating VEGFA and VEGFC
We further investigated whether VEGFA and VEGFC have an influence on the overexpression of MFSD4A-AS1-induced lymphatic metastasis and proliferation and migration of HUVECs. First, animal experiments in vivo showed that separately silencing VEGFA or VEGFC significantly reduced the number of tumor cells in lymph nodes from the MFSD4A-AS1-overexpressing mice group (Fig. 7A and B). Consistently, HUVECs tube formation and Transwell assays indicated that silencing VEGFA or VEGFC differentially attenuated the pro-mesh formation and migration ability of MFSD4A-AS1 overexpression in PTC cells (Fig. 7C and D). Collectively, our results demonstrated that VEGFA and VEGFC mediate the role of MFSD4A-AS1 overexpression in promoting lymphatic metastasis and proliferation and migration of HUVECs in vivo and in vitro in PTC.
Figure 7.
MFSD4A-AS1 promotes lymphatic metastasis and proliferation and migration of HUVECs by upregulating VEGFA and VEGFC. (A) H & E staining analysis of tumors in lymph nodes from the indicated mice group. (B) The number of tumor cells in the tumor areas of lymph nodes from the indicated mice group. *P < 0.05. (C) The effect of silencing VEGFA or VEGFC in MFSD4A-AS1 overexpression PTC cells on tube formation ability of HUVECs in tube formation assay. Each bar represents the mean values ± s.d. of three independent experiments. *P < 0.05. (D) The effect of silencing VEGFA or VEGFC in MFSD4A-AS1 overexpression PTC cells on migration ability of HUVECs in the Transwell assay. Each bar represents the mean values ± s.d. of three independent experiments. *P < 0.05.
MFSD4A-AS1 promotes lymphatic metastasis, proliferation and migration of HUVECs by partially activating TGF-β signaling
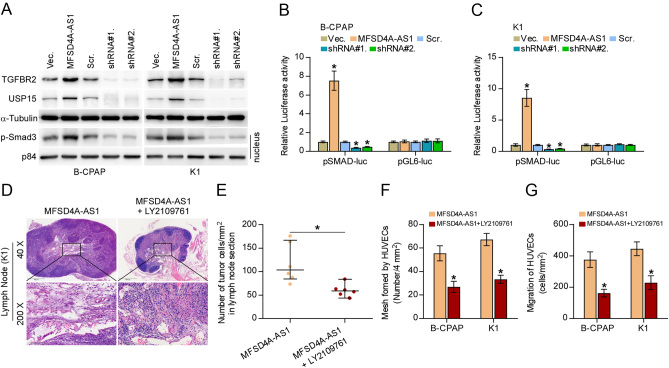
Fang and colleagues have reported that miR-30c-2-3p targeted TGFBR2 and USP15 in non-small cell lung cancer (NSCLC) cells, leading to the inactivation of TGF-β signaling (Fang et al. 2019). Of note, accumulating studies have reported that constitutive activation of TGF-β signaling plays a crucial role in lymphatic metastasis of tumors by inducing VEGFC expression (Liu et al. 2014, Huang et al. 2017, Shu et al. 2017), including PTC (He et al. 2018a ). Importantly, inhibition of TGF-β signaling activity effectively suppressed lymphatic metastasis of NSCLC (Salvo et al. 2014), suggesting the potential value of therapeutic strategy targeting TGF-β signaling in cancer. Therefore, we surmised that TGF-β signaling might mediate the role of MFSD4A-AS1 in promoting lymphangiogenesis of HUVECs given the finding that MFSD4A-AS1 inhibited miR-30c-2-3p expression in PTC cells (Fig. 6B), as well as the partial role of miR-30c-2-3p in reversing the effect of MFSD4A-AS1 overexpression on lymphangiogenesis of HUVECs. First, our results demonstrated that upregulating MFSD4A-AS1 not only elevated TGFBR2 and USP15 expression (Fig. 8A) but also increased nuclear pSMAD3 expression and transcriptional activity of the TGF-β/Smad-responsive luciferase reporter plasmid CAGA12 (Fig. 8A, B and C). Conversely, silencing MFSD4A-AS1 yielded an opposite effect (Fig. 8A, B and C). Of note, only miR-30c-2-3p agomir significantly reversed the effect of MFSD4A-AS1 overexpression on increased expression of TGFBR2 and USP15, as well as activation of TGF-β signaling (Supplementary Fig. 5A, B and C), and neither miR-145-3p nor miR-139-5p had an effect on the activity of TGF-β signaling (Supplementary Fig. 5D, E and F). Furthermore, either silencing TGFBR2 or USP15 or blocking activity of TGF-β signaling using LY2109761, a novel selective TGF-β receptor type I/II dual inhibitor (Melisi et al. 2008), differentially abrogated the stimulatory effect of MFSD4A-AS1 on invasive and migration abilities of PTC cells (Supplementary Fig. 6A and B). In turn, TβR1 TD, a constitutively active mutant TGFBRI plasmid containing a substitution of threonine 204 with aspartic acid (T204D) that leads to constitutive activation of TGF-β signaling in the absence of TGF-β stimulation (53, 54), significantly reversed the inhibitory effect of silencing MFSD4A-AS1 on invasive and migration capabilities of PTC cells (Supplementary Fig. 6C and D). Importantly, LY2109761 dramatically abrogated the pro-lymphatic metastasis role of MFSD4A-AS1-overexpression in vivo (Fig. 8D and E), and the pro-mesh formation of and migration ability of HUVECs in vitro in PTC cells (Fig. 8F and G). Collectively, our results showed that MFSD4A-AS1 promotes lymphatic metastasis, invasion and migration of PTC cells at least partially by activating TGF-β signaling.
Figure 8.
MFSD4A-AS1 promotes lymphatic metastasis, invasion and migration of PTC cells partially by activating TGF-β signaling. (A) Western blotting analysis of the effect of overexpression or silencing MFSD4A-AS1 on TGFBR2, USP15 and nuclear translocation of phosphorylated Smad3 (p-Smad3) in the indicated PTC cells. α-Tubulin and p84 served as the cytoplasmic and nuclear loading control, respectively. (B and C) The effect of overexpression or silencing MFSD4A-AS1 on TGF-β/Smad-responsive luciferase reporter in the indicated cells. Error bars represent the mean ± s.d. of three independent experiments. *P < 0.05. (D) H & E staining analysis of tumors in lymph nodes from the indicated mice group. (E) The number of tumor cells in the tumor areas of lymph nodes from the indicated mice group. *P < 0.05. (F) The effect of TGF-β inhibitor LY2109761 in MFSD4A-AS1 overexpression PTC cells on tube formation ability of HUVECs in tube formation assay. Each bar represents the mean values ± s.d. of three independent experiments. *P < 0.05. (G) The effect of TGF-β inhibitor LY2109761 in MFSD4A-AS1 overexpression PTC cells on migration ability of HUVECs in the Transwell assay. Each bar represents the mean values ± s.d. of three independent experiments. *P < 0.05.
Discussion
The critical findings of the current study present novel dissection for the first time, to the best of our knowledge, into the crucial role of MFSD4A-AS1 as a ceRNA in lymphatic metastasis of PTC. Here, we reported that MFSD4A-AS1 was remarkably and specifically upregulated in PTC tissues with lymph node metastasis. Functional experiments revealed that upregulating MFSD4A-AS1 promoted, while silencing MFSD4A-AS1 suppressed lymphatic metastasis of PTC cells in vivo, and proliferation and migration of HUVECs and invasion and migration of PTC cells in vitro. Our results further clarified that upregulating MFSD4A-AS1 promoted lymphatic metastasis of PTC by sequestering miR-30c-2-3p, miR-145-3p and miR-139-5p to disrupt the miRNAs-mediated inhibition of VEGFA and VEGFC and inactivation of TGF-β signaling. Collectively, our results determine the oncogenic role and underlying mechanism of MFSD4A-AS1 in lymphatic metastasis of PTC.
Numerous studies have demonstrated that the dysreuglation or aberrant expression of lncRNAs significantly contributed to lymphatic metastasis in various cancer types (Chen et al. 2018, He et al. 2018b). In the context of PTC, lncRNAs have been extensively documented to be able to function as oncogenic or tumor-suppressive factors to promote progression and metastasis of PTC (Gou et al. 2018, Esposito et al. 2019, Zhuang et al. 2019). The prime issue of PTC is its high avidity to metastasize to lymph node in which lncRNAs play an important role (Xia et al. 2017, Feng et al. 2019). Our previous study has shown that lncRNA MAPK8IP1P2 inhibited lymphatic metastasis of PTC by activating Hippo signaling via sponging miR-146b-3p (Liu et al. 2020). However, the clinical significance and functional role of MFSD4A-AS1 in PTC, especially in lymph node metastatic PTC, remain blank. In this study, our results demonstrated that there was no statistical difference in MFSD4A-AS1 expression between PTC tissues without lymph node metastasis and ANT, including in the T1–2 and T3–4 subgroups. Importantly, MFSD4A-AS1 was specifically and significantly upregulated in PTC tissues with lymph node metastasis compared with that in ANT and PTC tissues without lymph node metastasis. Gain- and loss-of-function assays demonstrated that upregulating MFSD4A-AS1 promoted, while silencing MFSD4A-AS1 suppressed proliferation and migration of HUVECs and invasion and migration of PTC cells in vitro, and lymphatic metastasis of PTC cells in vivo. Based on these findings, our results for the first time provide experimental evidence with regard to the clinical significance of MFSD4A-AS1 in PTC, particularly shedding light on the critical role of MFSD4A-AS1 in lymphatic metastasis of PTC.
As a class of versatile ncRNA, lncRNAs have been extensively validated to exert their biological functions depending on the subcellular localization of the lncRNAs, such as interacting with DNA within the nucleus as enhancers to regulate the target genes transcription of, as scaffold molecules to modulate protein/gene or protein/protein interactions (Ponting et al. 2009, Salehi et al. 2017). However, more and more attention focusing on the role of lncRNAs as ceRNAs by sponging target miRNAs to disrupt the miRNAs-mediated degradation of target genes has been made recently in cancer progression and metastasis (Karreth et al. 2011, Tay et al. 2011, Xu et al. 2019, Lang et al. 2020). Importantly, miRNA-mediated lncRNA/mRNA crosstalk plays a critical role in the development and metastasis of thyroid cancer (Credendino et al. 2019, Liu et al. 2019, Yao et al. 2019), including lymphatic metastasis (Feng et al. 2019, Zhuang et al. 2019, Li et al. 2020). In the current study, our results found that MFSD4A-AS1 functioned as ceRNA to sequester miR-30c-2-3p, miR-145-3p and miR-139-5p to disrupt the miRNA-mediated inhibition of VEGFA and VEGFC on the one hand; on the other hand, MFSD4A-AS1 further activated TGF-β signaling by sponging miR-30c-2-3p to relieve the repressive effect of miR-30c-2-3p on TGFBR2 and USP15, which synergistically promoted lymphangiogenesis and lymphatic metastasis of PTC. Therefore, our findings unravel novel dual mechanisms by which MFSD4A-AS1 promoted lymphatic metastasis of PTC.
TGF-β signaling has been widely reported to be implicated in multiple biological processes, such as cell proliferation and differentiation and bone remodeling (Mohammad et al. 2009). Interestingly, TGF-β signaling exerts a complex and even paradoxical function in cancer scenario depending on the developmental stage of tumor: TGF-β signaling functions as a tumor suppressor to inhibit cell growth in early stages; whereas in later stages, TGF-β signaling plays an oncogenic role in promoting invasion and metastasis of tumor cells (Pickup et al. 2013). Furthermore, activation of TGF-β signaling has been demonstrated to expedite lymphatic metastasis of tumors via varying mechanisms (Liu et al. 2014, Huang et al. 2017, Shu et al. 2017), where TGF-β-induced dramatical upregulation of VEGFC in tumor cells was regarded as the primary contributor (Liu et al. 2014). Notably, a fundamental study from He et al. has reported that SLC35F2 promoted the proliferation, migration and lymphatic metastasis of PTC by activating TGF-β signaling (He et al. 2018a), elucidating the critical role of TGF-β signaling in lymphatic metastasis of thyroid cancer. Importantly, inhibition of TGF-β signaling activity effectively suppressed lymphatic metastasis of NSCLC (Salvo et al. 2014), implying that therapeutic strategy targeting TGF-β signaling holds favorable prospects for the treatment of lymphatic metastasis of cancer. In this study, our results demonstrated that MFSD4A-AS1 upregulated TGFBR2 and USP15, and activated TGF-β signaling by sponging miR-30c-2-3p. Importantly, reconstituting activity of TGF-β signaling using transfecting TβR1 TD dramatically reversed the inhibitory effect of silencing MFSD4A-AS1 on invasion and migration of PTC cells. Taken together, our results suggested that therapeutic avenue by inhibition of MFSD4A-AS1 holds a promising efficacy in lymphatic metastasis of PTC. However, more solid conclusion regarding the therapeutic efficacy of MFSD4A-AS1 inhibition in lymphatic metastasis of PTC is definitely worth further investigation through a series of animal and clinical trials.
In summary, our results demonstrate that MFSD4A-AS1 promotes lymphangiogenesis and lymphatic metastasis of PTC by sequestering miR-30c-2-3p, miR-145-3p and miR-139-5p to disrupt the miRNAs-mediated both inhibition of VEGFA and VEGFC expression and inactivation of TGF-β signaling. An in-depth understanding of the functional role and underlying mechanism by which MFSD4A-AS1 promotes lymphatic metastasis of PTC will facilitate to clarify the pathogenesis of lymphatic metastasis of PTC.
Supplementary Material
Declaration of interest
The authors declare no conflict of interest.
Funding
This study was funded by the National Natural Science Foundation of China (grant number 81972499), the Department of Science and Technology of Jilin Province, China (grant number 20200201181JC), the Finance Department of Jilin Province, China (grant number 2020SCZ51), and Jilin University, China (grant number 2020B12).
Author contribution statement
Liu Xiaoli and Sun Hui conceived the project and drafted the manuscript. Liu Xiaoli, Chunhai Zhang and Xiaomiao Wang conducted the experiments and contributed to the analysis of data. Can Cui and Hanwen Cui performed the animal experiments. Baishu Zhu and Anqi Chen analyzed the informatics data. Lu Zhang and Jingwei Xin performed IHC and the analysis of data. Qingfeng Fu contributed to the cell biology and molecular biology experiments. Liu Xiaoli and Gianlorenzo Dionigi edited and revised the manuscript. All authors discussed the results and approved the manuscript.
References
- Albores-Saavedra J, Henson DE, Glazer E, Schwartz AM.2007Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype--papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocrine Pathology 181–7. ( 10.1007/s12022-007-0002-z) [DOI] [PubMed] [Google Scholar]
- Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK.2012Thyroid cancer in Denmark 1943–2008, before and after iodine supplementation. International Journal of Cancer 1312360–2366. ( 10.1002/ijc.27497) [DOI] [PubMed] [Google Scholar]
- Burton JB, Priceman SJ, Sung JL, Brakenhielm E, An DS, Pytowski B, Alitalo K, Wu L.2008Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Research 687828–7837. ( 10.1158/0008-5472.CAN-08-1488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, He W, Huang J, Wang B, Li H, Cai Q, Su F, Bi J, Liu H, Zhang Bet al. 2018LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nature Communications 9 3826. ( 10.1038/s41467-018-06152-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Luo Y, He W, Zhao Y, Kong Y, Liu H, Zhong G, Li Y, Li J, Huang Jet al. 2020aExosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. Journal of Clinical Investigation 130404–421. ( 10.1172/JCI130892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Liu A, Lin Z, Wang B, Chai X, Chen S, Lu W, Zheng M, Cao T, Zhong Met al. 2020bDownregulation of the circadian rhythm regulator HLF promotes multiple-organ distant metastases in non-small cell lung cancer through PPAR/NF-kappab signaling. Cancer Letters 48256–71. ( 10.1016/j.canlet.2020.04.007) [DOI] [PubMed] [Google Scholar]
- Chen J, Liu A, Wang Z, Wang B, Chai X, Lu W, Cao T, Li R, Wu M, Lu Zet al. 2020cLINC00173.v1 promotes angiogenesis and progression of lung squamous cell carcinoma by sponging miR-511-5p to regulate VEGFA expression. Molecular Cancer 19 98. ( 10.1186/s12943-020-01217-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credendino SC, Bellone ML, Lewin N, Amendola E, Sanges R, Basu S, Sepe R, Decaussin-Petrucci M, Tinto N, Fusco Aet al. 2019A ceRNA circuitry involving the long noncoding RNA Klhl14-AS, Pax8, and Bcl2 drives thyroid carcinogenesis. Cancer Research 795746–5757. ( 10.1158/0008-5472.CAN-19-0039) [DOI] [PubMed] [Google Scholar]
- Dai Y, Ren D, Yang Q, Cui Y, Guo W, Lai Y, Du H, Lin C, Li J, Song Let al. 2017The TGF-beta signalling negative regulator PICK1 represses prostate cancer metastasis to bone. British Journal of Cancer 117685–694. ( 10.1038/bjc.2017.212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Wu Z, Lang C, Zhang X, He S, Yang Q, Guo W, Lai Y, Du H, Peng Xet al. 2019Copy number gain of ZEB1 mediates a double-negative feedback loop with miR-33a-5p that regulates EMT and bone metastasis of prostate cancer dependent on TGF-beta signaling. Theranostics 96063–6079. ( 10.7150/thno.36735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R, Esposito D, Pallante P, Fusco A, Ciccodicola A, Costa V.2019Oncogenic properties of the antisense lncRNA COMET in BRAF- and RET-driven papillary thyroid carcinomas. Cancer Research 792124–2135. ( 10.1158/0008-5472.CAN-18-2520) [DOI] [PubMed] [Google Scholar]
- Fang L, Wu S, Zhu X, Cai J, Wu J, He Z, Liu L, Zeng M, Song E, Li Jet al. 2019MYEOV functions as an amplified competing endogenous RNA in promoting metastasis by activating TGF-beta pathway in NSCLC. Oncogene 38896–912. ( 10.1038/s41388-018-0484-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Zhou Q, Yi H, Ma S, Li D, Xu Y, Wang J, Yin S.2019A novel lncRNA n384546 promotes thyroid papillary cancer progression and metastasis by acting as a competing endogenous RNA of miR-145-5p to regulate AKT3. Cell Death and Disease 10 433. ( 10.1038/s41419-019-1637-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Q, Gao L, Nie X, Pu W, Zhu J, Wang Y, Liu X, Tan S, Zhou JK, Gong Yet al. 2018Long noncoding RNA AB074169 inhibits cell proliferation via modulation of KHSRP-mediated CDKN1a expression in papillary thyroid carcinoma. Cancer Research 784163–4174. ( 10.1158/0008-5472.CAN-17-3766) [DOI] [PubMed] [Google Scholar]
- Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, Maurer MS, Mciver B, Mullan BP, Oberg ALet al. 2002Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World Journal of Surgery 26879–885. ( 10.1007/s00268-002-6612-1) [DOI] [PubMed] [Google Scholar]
- He J, Jin Y, Zhou M, Li X, Chen W, Wang Y, Gu S, Cao Y, Chu C, Liu Xet al. 2018aSolute carrier family 35 member F2 is indispensable for papillary thyroid carcinoma progression through activation of transforming growth factor-beta type I receptor/apoptosis signal-regulating kinase 1/mitogen-activated protein kinase signaling axis. Cancer Science 109642–655. ( 10.1111/cas.13478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Zhong G, Jiang N, Wang B, Fan X, Chen C, Chen X, Huang J, Lin T.2018bLong noncoding RNA BLACAT2 promotes bladder cancer-associated lymphangiogenesis and lymphatic metastasis. Journal of Clinical Investigation 128861–875. ( 10.1172/JCI96218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K.2002Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. Journal of the National Cancer Institute 94819–825. ( 10.1093/jnci/94.11.819) [DOI] [PubMed] [Google Scholar]
- Huang SC, Wei PC, Hwang-Verslues WW, Kuo WH, Jeng YM, Hu CM, Shew JY, Huang CS, Chang KJ, Lee EYet al. 2017TGF-beta1 secreted by Tregs in lymph nodes promotes breast cancer malignancy via up-regulation of IL-17RB. EMBO Molecular Medicine 91660–1680. ( 10.15252/emmm.201606914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K.1996A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO Journal 15290–298. ( 10.1002/j.1460-2075.1996.tb00359.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karreth FA, Tay Y, Perna D, Ala U, Tan SM, Rust AG, Denicola G, Webster KA, Weiss D, Perez-Mancera PAet al. 2011In vivo identification of tumor- suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell 147382–395. ( 10.1016/j.cell.2011.09.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Dai Y, Wu Z, Yang Q, He S, Zhang X, Guo W, Lai Y, Du H, Wang Het al. 2020SMAD3/SP1 complex-mediated constitutive active loop between lncRNA PCAT7 and TGF-beta signaling promotes prostate cancer bone metastasis. Molecular Oncology 14808–828. ( 10.1002/1878-0261.12634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Yin C, Lin K, Li Y, Yang Q, Wu Z, Du H, Ren D, Dai Y, Peng X.2021m6 A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clinical and Translational Medicine 11 e426. ( 10.1002/ctm2.426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Chai L, Yu X, Song Y, Zhu X, Fan S, Jiang W, Qiao T, Tong J, Liu Set al. 2020The HOTAIRM1/miR-107/TDG axis regulates papillary thyroid cancer cell proliferation and invasion. Cell Death and Disease 11 227. ( 10.1038/s41419-020-2416-1) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li X, Liu F, Lin B, Luo H, Liu M, Wu J, Li C, Li R, Zhang X, Zhou Ket al. 2017miR150 inhibits proliferation and tumorigenicity via retarding G1/S phase transition in nasopharyngeal carcinoma. International Journal of Oncology 501097–1108. ( 10.3892/ijo.2017.3909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Li L, Zhang XX, Wan DY, Xi BX, Hu Z, Ding WC, Zhu D, Wang XL, Wang Wet al. 2014SIX1 promotes tumor lymphangiogenesis by coordinating TGFbeta signals that increase expression of VEGF-C. Cancer Research 745597–5607. ( 10.1158/0008-5472.CAN-13-3598) [DOI] [PubMed] [Google Scholar]
- Liu X, Fu Q, Li S, Liang N, Li F, Li C, Sui C, Dionigi G, Sun H.2019LncRNA FOXD2-AS1 functions as a competing endogenous RNA to regulate tert expression by sponging miR-7-5p in thyroid cancer. Frontiers in Endocrinology (Lausanne) 10 207. ( 10.3389/fendo.2019.00207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Fu Q, Bian X, Fu Y, Xin J, Liang N, Li S, Zhao Y, Fang L, Li Cet al. 2020Long non-coding RNA MAPK8IP1P2 inhibits lymphatic metastasis of thyroid cancer by activating hippo signaling via sponging miR-146b-3p. Frontiers in Oncology 10 600927. ( 10.3389/fonc.2020.600927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Xing M.2016Recent incidences and differential trends of thyroid cancer in the USA. Endocrine-Related Cancer 23313–322. ( 10.1530/ERC-15-0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, Abbruzzese JL, Chiao PJ.2008LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Molecular Cancer Therapeutics 7829–840. ( 10.1158/1535-7163.MCT-07-0337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Feng Z, Edens BM, Yang B, Shi H, Sze CC, Hong BT, Su SC, Cantu JA, Topczewski Jet al. 2015Non-aggregating tau phosphorylation by cyclin-dependent kinase 5 contributes to motor neuron degeneration in spinal muscular atrophy. Journal of Neuroscience 356038–6050. ( 10.1523/JNEUROSCI.3716-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad KS, Chen CG, Balooch G, Stebbins E, Mckenna CR, Davis H, Niewolna M, Peng XH, Nguyen DH, Ionova-Martin SSet al. 2009Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS One 4 e5275. ( 10.1371/journal.pone.0005275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Conde C, Grana-Castro O, Martin-Serrano G, Martinez-Montes ÁM, Zarzuela E, Munoz J, Torres-Perez R, Pita G, Cordero-Barreal A, Leandro-Garcia LJet al. 2020Hsa-miR-139-5p is a prognostic thyroid cancer marker involved in HNRNPF-mediated alternative splicing. International Journal of Cancer 146521–530. ( 10.1002/ijc.32622) [DOI] [PubMed] [Google Scholar]
- Nakamura ES, Koizumi K, Kobayashi M, Saiki I.2004Inhibition of lymphangiogenesis-related properties of murine lymphatic endothelial cells and lymph node metastasis of lung cancer by the matrix metalloproteinase inhibitor MMI270. Cancer Science 9525–31. ( 10.1111/j.1349-7006.2004.tb03166.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson s.d.2003Insights into the mechanisms of lymph node metastasis. Cancer 98413–423. ( 10.1002/cncr.11464) [DOI] [PubMed] [Google Scholar]
- Peng P, Chen T, Wang Q, Zhang Y, Zheng F, Huang S, Tang Y, Yang C, Ding W, Ren Det al. 2019Decreased miR-218-5p levels as a serum biomarker in bone metastasis of prostate. Cancer and Oncology Research Treat 42165–185. ( 10.1159/000495473) [DOI] [PubMed] [Google Scholar]
- Pickup M, Novitskiy S, Moses HL.2013The roles of TGFbeta in the tumour microenvironment. Nature Reviews. Cancer 13788–799. ( 10.1038/nrc3603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP, Oliver PL, Reik W.2009Evolution and functions of long noncoding RNAs. Cell 136629–641. ( 10.1016/j.cell.2009.02.006) [DOI] [PubMed] [Google Scholar]
- Ren D, Wang M, Guo W, Zhao X, Tu X, Huang S, Zou X, Peng X.2013Wild-type p53 suppresses the epithelial-mesenchymal transition and stemness in PC-3 prostate cancer cells by modulating miR145. International Journal of Oncology 421473–1481. ( 10.3892/ijo.2013.1825) [DOI] [PubMed] [Google Scholar]
- Ren D, Lin B, Zhang X, Peng Y, Ye Z, Ma Y, Liang Y, Cao L, Li X, Li Ret al. 2017aMaintenance of cancer stemness by miR-196b-5p contributes to chemoresistance of colorectal cancer cells via activating STAT3 signaling pathway. Oncotarget 849807–49823. ( 10.18632/oncotarget.17971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Yang Q, Dai Y, Guo W, Du H, Song L, Peng X.2017bOncogenic miR-210-3p promotes prostate cancer cell EMT and bone metastasis via NF-kappaB signaling pathway. Molecular Cancer 16 117. ( 10.1186/s12943-017-0688-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Dai Y, Yang Q, Zhang X, Guo W, Ye L, Huang S, Chen X, Lai Y, Du Het al. 2019Wnt5a induces and maintains prostate cancer cells dormancy in bone. Journal of Experimental Medicine 216428–449. ( 10.1084/jem.20180661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, Pytowski B, Skobe M.2006Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Research 662650–2657. ( 10.1158/0008-5472.CAN-05-1843) [DOI] [PubMed] [Google Scholar]
- Saiselet M, Gacquer D, Spinette A, Craciun L, Decaussin-Petrucci M, Andry G, Detours V, Maenhaut C.2015New global analysis of the microRNA transcriptome of primary tumors and lymph node metastases of papillary thyroid cancer. BMC Genomics 16 828. ( 10.1186/s12864-015-2082-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi S, Taheri MN, Azarpira N, Zare A, Behzad-Behbahani A.2017State of the art technologies to explore long non-coding RNAs in cancer. Journal of Cellular and Molecular Medicine 213120–3140. ( 10.1111/jcmm.13238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvo E, Garasa S, Dotor J, Morales X, Pelaez R, Altevogt P, Rouzaut A.2014Combined targeting of TGF-beta1 and integrin beta3 impairs lymph node metastasis in a mouse model of non-small-cell lung cancer. Molecular Cancer 13 112. ( 10.1186/1476-4598-13-112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu YJ, Bao RF, Jiang L, Wang Z, Wang XA, Zhang F, Liang HB, Li HF, Ye YY, Xiang SSet al. 2017MicroRNA-29c-5p suppresses gallbladder carcinoma progression by directly targeting CPEB4 and inhibiting the MAPK pathway. Cell Death and Differentiation 24445–457. ( 10.1038/cdd.2016.146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG.2014Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nature Reviews. Cancer 14159–172. ( 10.1038/nrc3677) [DOI] [PubMed] [Google Scholar]
- Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, Karreth F, Poliseno L, Provero P, Di Cunto Fet al. 2011Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 147344–357. ( 10.1016/j.cell.2011.09.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ren D, Guo W, Huang S, Wang Z, Li Q, Du H, Song L, Peng X.2016N-cadherin promotes epithelial-mesenchymal transition and cancer stem cell-like traits via ErbB signaling in prostate cancer cells. International Journal of Oncology 48595–606. ( 10.3892/ijo.2015.3270) [DOI] [PubMed] [Google Scholar]
- Wu N, Ren D, Li S, Ma W, Hu S, Jin Y, Xiao S.2018RCC2 over-expression in tumor cells alters apoptosis and drug sensitivity by regulating Rac1 activation. BMC Cancer 18 67. ( 10.1186/s12885-017-3908-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Wang C, Ni X, Ni Z, Dong Y, Zhan W.2017NONHSAT076754 aids ultrasonography in predicting lymph node metastasis and promotes migration and invasion of papillary thyroid cancer cells. Oncotarget 82293–2306. ( 10.18632/oncotarget.13725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Meng Q, Li X, Yang H, Xu J, Gao N, Sun H, Wu S, Familiari G, Relucenti M, Zhu H, Wu J, Chen R.2019Long Noncoding RNA MIR17HG Promotes Colorectal Cancer Progression via miR-17-5p. Cancer Res 794882–4895. ( 10.1158/0008-5472.can-18-3880) [DOI] [PubMed] [Google Scholar]
- Yao N, Fu Y, Chen L, Liu Z, He J, Zhu Y, Xia T, Wang S.2019Long non-coding RNA NONHSAT101069 promotes epirubicin resistance, migration, and invasion of breast cancer cells through NONHSAT101069/miR-129-5p/Twist1 axis. Oncogene 387216–7233. ( 10.1038/s41388-019-0904-5) [DOI] [PubMed] [Google Scholar]
- Yu XM, Lo CY, Chan WF, Lam KY, Leung P, Luk JM.2005Increased expression of vascular endothelial growth factor C in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clinical Cancer Research 118063–8069. ( 10.1158/1078-0432.CCR-05-0646) [DOI] [PubMed] [Google Scholar]
- Yu XM, Lo CY, Lam AK, Leung P, Luk JM.2008Serum vascular endothelial growth factor C correlates with lymph node metastases and high-risk tumor profiles in papillary thyroid carcinoma. Annals of Surgery 247483–489. ( 10.1097/SLA.0b013e31815fa447) [DOI] [PubMed] [Google Scholar]
- Zhang X, Ren D, Guo L, Wang L, Wu S, Lin C, Ye L, Zhu J, Li J, Song Let al. 2017aThymosin beta 10 is a key regulator of tumorigenesis and metastasis and a novel serum marker in breast cancer. Breast Cancer Research 19 15. ( 10.1186/s13058-016-0785-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Lin B, Chai X, Li R, Liao Y, Deng X, Liu Q, Yang W, Cai Yet al. 2017bPhospholipid phosphatase 4 promotes proliferation and tumorigenesis, and activates Ca2+-permeable Cationic Channel in lung carcinoma cells. Molecular Cancer 16 147. ( 10.1186/s12943-017-0717-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ren D, Wu X, Lin X, Ye L, Lin C, Wu S, Zhu J, Peng X, Song L.2018miR-1266 contributes to pancreatic cancer progression and chemoresistance by the STAT3 and NF-kappaB signaling pathways. Molecular Therapy. Nucleic Acids 11142–158. ( 10.1016/j.omtn.2018.01.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shen YP, Li JG, Chen G.2019Clinical feasibility of imaging with indocyanine green combined with carbon nanoparticles for sentinel lymph node identification in papillary thyroid microcarcinoma. Medicine (Baltimore) 98 e16935. ( 10.1097/MD.0000000000016935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Tong H, Ding Y, Wu L, Cai J, Si Y, Zhang H, Shen M.2019Long noncoding RNA ABHD11-AS1 functions as a competing endogenous RNA to regulate papillary thyroid cancer progression by miR-199a-5p/SLC1A5 axis. Cell Death and Disease 10 620. ( 10.1038/s41419-019-1850-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a