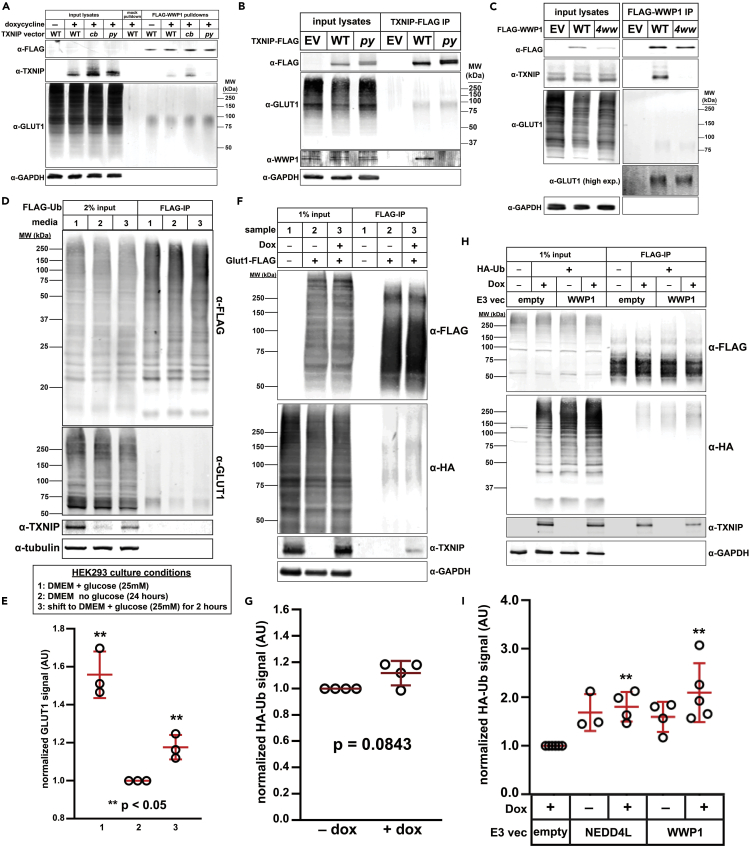
Figure 5.
TXNIP is dispensable for GLUT1 ubiquitin modification
(A) HeLa cells stably expressing GLUT1-GFP were stably transfected with either empty vector (pINDUCER20) or vector expressing wild type, clathrin-binding mutant (cb), or py motif mutant (py) TXNIP under the control of Tet-on gene expression system. 1 μg/ml doxycycline was added to induce expression of TXNIP for 24 h prior to collection of cell lysate. Cell lysates were incubated with recombinant WWP1-FLAG at 4°C overnight then WWP1-FLAG was pulled down using αFLAG magnetic beads. Elution was performed using FLAG peptide. Lysates and eluates were resolved by SDS-PAGE and analyzed by immunoblot. Immunoblotting of GAPDH was performed as a loading control.
(B) HeLa cells stably expressing GLUT1-GFP were transiently transfected with either a wild type or py mutant TXNIP-FLAG expression plasmid. When cells reached 100% confluence, they were collected in lysis buffer and incubated with αFLAG magnetic beads for 1 h at 4°C with rotation. TXNIP-FLAG was eluted with FLAG peptide and samples were resolved by SDS-PAGE then analyzed by immunoblot. GAPDH was used as a loading control.
(C) HeLa cells stably expressing GLUT1-GFP and a dox-inducible clathrin-binding mutant TXNIP were transiently transfected with either wild-type WWP1-FLAG or a mutant WWP1-FLAG with all 4 ww domains mutated. TXNIPCB was induced with 1 μg/ml doxycycline 24 h before collecting lysates. Cells were then collected in lysis buffer and lysates were incubated with αFLAG magnetic beads for 1 h at 4°C with rotation. WWP1 was eluted using FLAG peptide and samples were resolved by SDS-PAGE and analyzed by immunoblot. GAPDH was used as a loading control.
(D) HEK293T cells stably expressing FLAG-Ub were split into either 1) regular 25 mM glucose DMEM media, 2) DMEM media with no glucose, or 3) no glucose DMEM media and switched to 25 mM glucose media 2 h before collection. All cells were transiently transfected with a GLUT1-GFP expression plasmid. When cells reached 100% confluency, sample 3 cells were switched to high glucose (25 mM) DMEM media and lysates were collected 2 h later then incubated with magnetic FLAG affinity beads for 1 h at 4°C with rotation. FLAG-Ub was eluted using FLAG peptide; samples were resolved by SDS-PAGE, and analyzed by immunoblot. α-Tubulin was used as a loading control.
(E) Quantification of the eluate GLUT1 signal for three biological replicates (n = 3) of the experiment shown in (D). ∗∗ indicates a significant difference (p < 0.05) compared to the no glucose condition (lane 2).
(F) txnip knockout HeLa cells stably expressing constitutive GLUT1-FLAG and a dox-inducible TXNIP expression plasmid were transiently transfected with HA-Ub. 24 hours before collecting lysates, cells were either mock-treated (sample 2) or treated with 1 µg/ml doxycycline (sample 3) to induce TXNIP expression. As a control, HeLa cells with a stably integrated empty vector (i.e., endogenous TXNIP but no GLUT1-FLAG expression) were also analyzed (sample 1). Lysates were incubated with magnetic αFLAG affinity beads for 1 h at 4°C with rotation and eluted using FLAG peptide. Samples were resolved by SDS-PAGE and analyzed by immunoblot. GAPDH was used as a loading control.
(G) Quantification of the eluate HA-Ub signal in four biological replicates (n = 4) of the experiment shown in (F).
(H) HeLa cells stably expressing constitutive GLUT1-FLAG and dox-inducible TXNIP vectors were transiently transfected with either empty vector, HA-Ub, and/or WWP1 as indicated in the figure. 24 hours after inducing TXNIP with 1 μg/ml doxycyxline, cells were collected and lysed. Lysates were incubated with magnetic αFLAG affinity beads for 1 h at 4°C with rotation. GLUT1-FLAG was eluted with FLAG peptide and samples were resolved by SDS-PAGE then analyzed by immunoblot. GAPDH was used as a loading control.
(I) Quantification of HA-Ub signal for at least three biological replicates (n ≥ 3) of the experiments shown in (H). Double asterisk (∗∗) indicates a significant difference (p < 0.05) compared to the empty vector control. All p-values were measured using a two sample Student’s t-Test in Microsoft Excel. A P value < 0.05 was considered statistically significant and is indicated by ∗∗. Data are represented as mean +/- SEM.