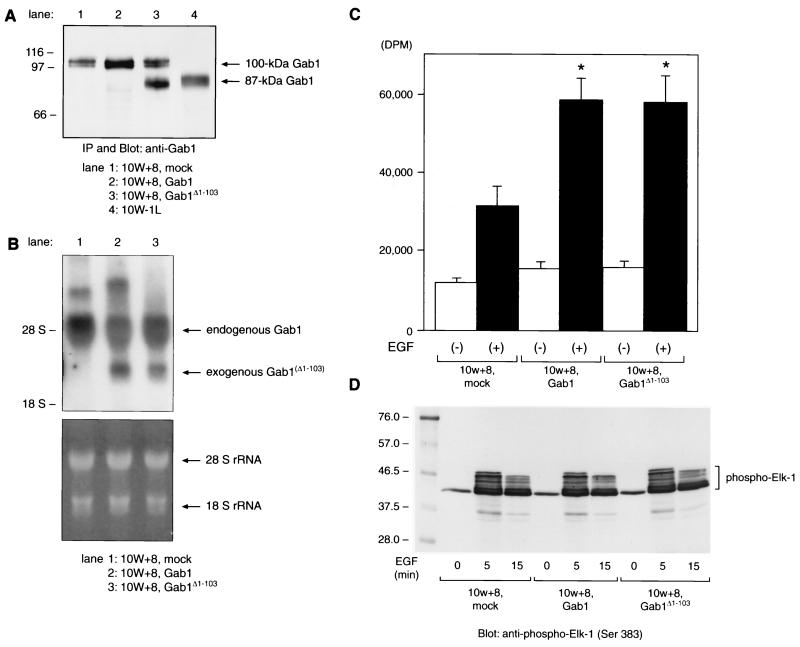
FIG. 7.
Exogenous expression of Gab1 or Gab1Δ1-103 in 10W+8 cells enhances the mitogenic response to EGF stimulation without an upregulated p44/42 MAPK activation. (A) Immunoprecipitation (IP) and Western analysis of Gab1- or Gab1Δ1-103-overexpressing 10W+8. Gab1Δ1-103 expressed in 10W+8 (lane 3) comigrated with 87-kDa Gab1 from 10W−1L (lane 4). (B) Northern analysis of 10W+8 cells exogenously expressing Gab1 or Gab1Δ1-103. Exogenously expressed mRNA of Gab1 (lane 2, 2.7 kb) or Gab1Δ1-103 (lane 3, 2.7 kb) lacking most of the 5′- and 3′-untranslated region was distinct from endogenous Gab1 mRNA (5.2 kb). The lower panel shows equal RNA loading onto each lane and the integrity of RNA samples by visualization of 28S/18S rRNA with ethidium bromide staining and UV exposure. (C) [3H]thymidine incorporation assay was performed using mock-transfected 10W+8 and Gab1/Gab1Δ1-103-overexpressing 10W+8 cells. Cells were serum starved for 20 h and incubated with or without 50 ng of EGF/ml for a further 24 h in the presence of 1 μCi of [3H]thymidine/well. Both Gab1 and Gab1Δ1-103 overexpression significantly potentiated the mitogenic response to EGF stimulation. ∗, P < 0.05 versus mock-transfected 10W+8. (D) EGF-dependent activation of p44/42 (ERK1/2) MAPK in 10W+8 cells was not significantly modulated by Gab1 or Gab1Δ1-103 overexpression. Cells were serum starved for 20 h and left unstimulated or stimulated with 100 ng of EGF/ml for 5 and 15 min. Cell lysates containing 200 μg of protein were incubated with anti-phospho-MAPK antibody. Immunoprecipitated phospho-MAPK was then reacted in vitro with Elk-1 as a substrate. MAPK-phosphorylated Elk-1 was analyzed by SDS–10% PAGE and anti-phospho-Elk-1 blotting. Some of the Ser381-phosphorylated Elk-1 showed various degrees of delayed migration on SDS-PAGE.