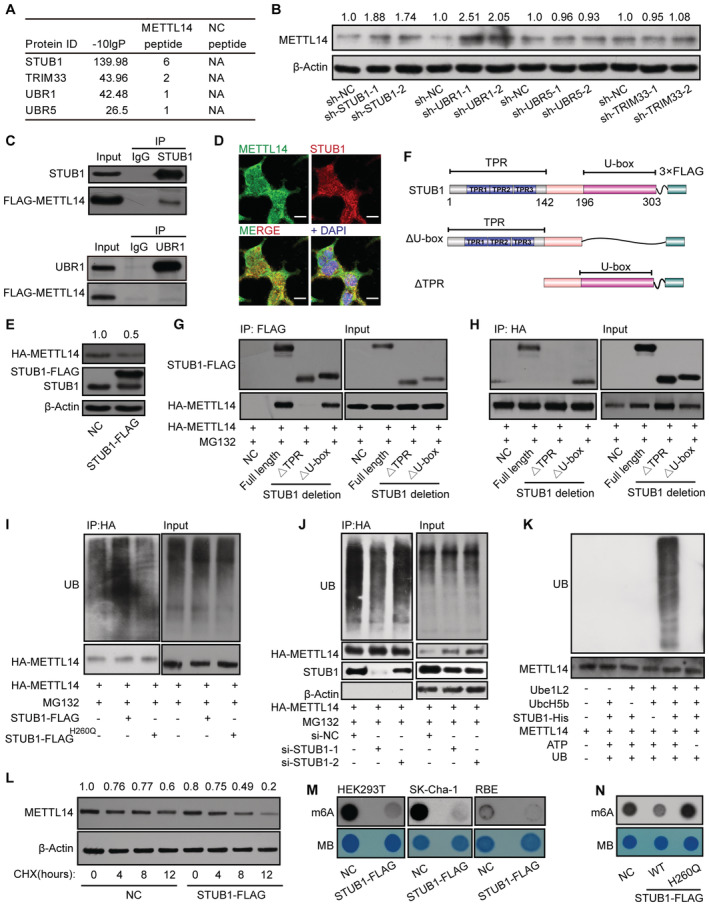
Figure 3. STUB1 is the E3 ubiquitin ligase to promote METTL14 ubiquitination.
-
AList of E3 ligases in the METTL14 interactome identified by MS.
-
BImmunoblots showing METTL14 protein levels in cells with shRNA knockdown of UBR1, STUB1, UBR5, or TRIM33. The METTL14 /β‐actin densitometric ratio was recorded by ImageJ.
-
CCo‐IP assays between FLAG‐METTL14 and STUB1 or URB1.
-
DImmunofluorescence of METTL14 (green) colocalized with that of STUB1 (red) in HEK293T cells. Nuclei were stained with DAPI (blue). Scale bar, 20 μm.
-
EImmunoblots showing the protein level of METTL14 after transfection with a plasmid encoding STUB1. β‐actin was used as the loading control. The HA‐METTL14/β‐actin densitometric ratio was recorded by ImageJ.
-
FSchematic diagram of STUB1‐domain‐deletion mutants.
-
G, HHA‐METTL14 Co‐IP with FLAG‐tagged STUB1‐domain‐deletion mutants.
-
IImmunoblots showing METTL14 ubiquitination assays under ectopic expression of STUB1 or the STUB1 H260Q mutant, which encodes a protein that lacks E3 ligase activity.
-
JImmunoblots showing METTL14 ubiquitination assays under STUB1 siRNA knockdown.
-
KIn vitro ubiquitination assays showing that purified METLL14 is ubiquitinated by purified STUB1‐His. Ube1L2 and UbcH5b were the E1 and E2 enzymes, respectively, in the ubiquitination system. ATP and ubiquitin (UB) were also added to the assay.
-
LImmunoblots showing CHX assay in HEK293T cells. Negative control (NC; no transfected cells) and STUB1‐FLAG plasmid‐transfected HEK293T cells were exposed to 100 μg/ml CHX for 0, 4, 8, or 12 h. β‐actin was used as the loading control. The METTL14/β‐actin densitometric ratio was recorded by ImageJ.
-
M, NDot blot assays showing that enforced expression of wild‐type STUB1, but not the STUB1 H260Q mutant, significantly decreases total m6A levels.
Source data are available online for this figure.
