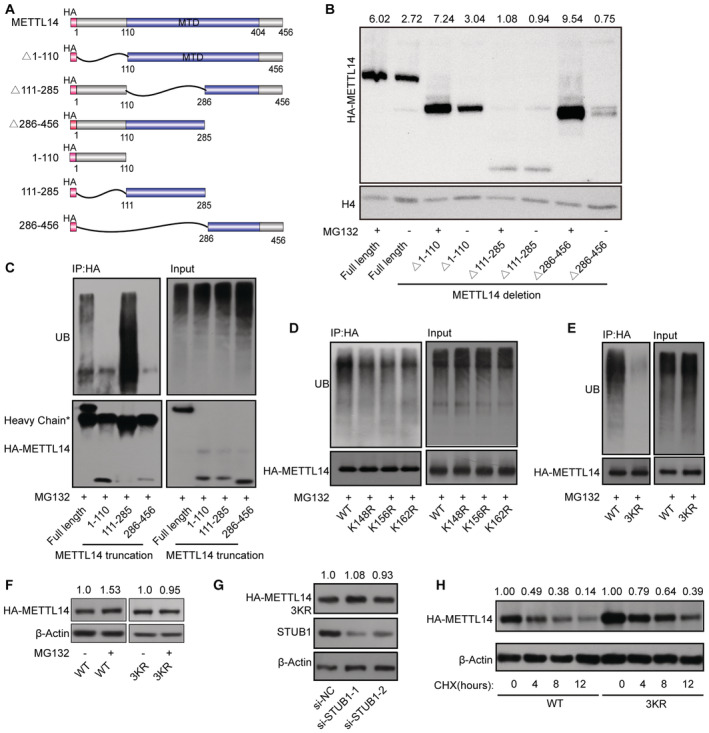
Figure 4. K148, K156, and K162 are the main sites of METTL14 ubiquitination by STUB1.
-
ASchematic diagram of METTL14‐domain‐deletion mutants. All mutants were HA‐tagged.
-
BImmunoblots showing the abundance of METTL14 truncated mutants with or without 10 μM MG132 treatment. Histone H4 was used as the loading control. The HA‐METTL14/β‐actin densitometric ratio was recorded by ImageJ.
-
CMETTL14 ubiquitination, as detected by IP of METTL14 truncated mutants with anti‐HA antibody and immunoblotting with anti‐ubiquitin (anti‐Ub) antibody. Accumulation of Ub and METTL14 was confirmed in whole‐cell lysates.
-
D, EMETTL14 ubiquitination, as detected by IP of a series of mutants with lysine (K)‐to‐arginine (R) substitutions in the aa 111–285 portion of the protein. The accumulation of Ub and METTL14 was confirmed in the whole‐cell lysates. WT, wild type; 3KR, K148, K156, and K162 all mutated to R.
-
FImmunoblots showing the levels of METTL14 protein after transfection with METTL14‐3KR mutant plasmids with or without 10 μM MG132. β‐actin was used as the loading control. The HA‐METTL14/β‐actin densitometric ratio was recorded by ImageJ.
-
GImmunoblots showing the protein level of METTL14 after transfection with METTL14‐3KR mutant plasmids under STUB1 knockdown. β‐actin was used as the loading control. The HA‐METTL14/β‐actin densitometric ratio was recorded by ImageJ.
-
HImmunoblots showing HEK293T cells in response to CHX treatment. HA‐METTL14 and HA‐METTL14‐3KR plasmid‐transfected HEK293T cells were exposed to 100 μg/ml CHX for 0, 4, 8, or 12 h. β‐actin was used as the loading control. The HA‐METTL14/β‐actin densitometric ratio was recorded by ImageJ.
Source data are available online for this figure.
