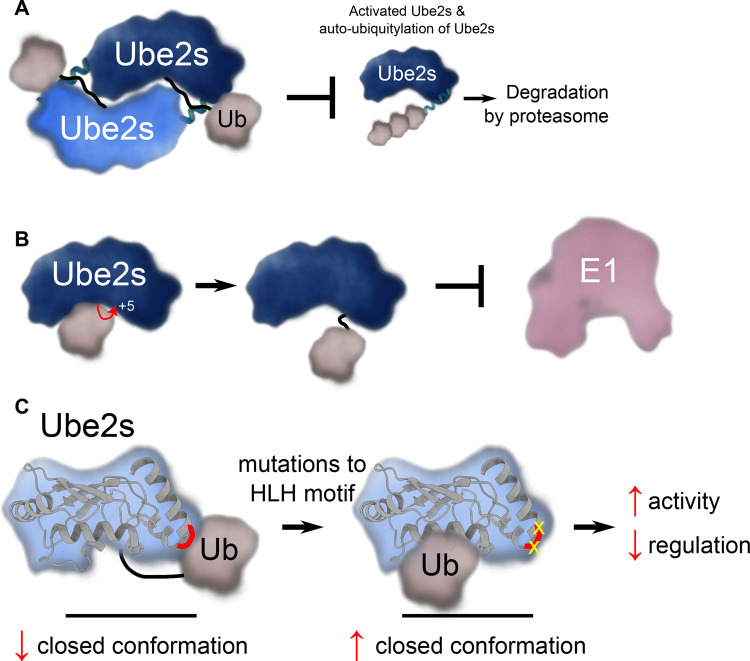
Figure 3. Regulation of Ube2s.
(A) In the absence of recruitment by the APC/C E3 ligase, Ube2s can form a dimer that prevents the conjugated ubiquitin from binding Ube2s in the catalytically active closed conformation. The dimerisation blocks interaction with the E1 enzyme and Ube2s-catalysed ubiquitin transfer, including autoubiquitylation of Ube2s. (B) Ube2s can autoubiquitylate on a position five amino acids from its active site and thereby prevent Ube2s from being charged with ubiquitin by an E1 enzyme. (C) The helix-loop-helix region of Ube2s (indicated in red) has acidic residues that bind ubiquitin in a non-catalytic conformation to regulate its activity in the absence of the APC/C E3 ligase. Mutations (in yellow) that disrupt this site result in heightened activity, but decrease regulation.