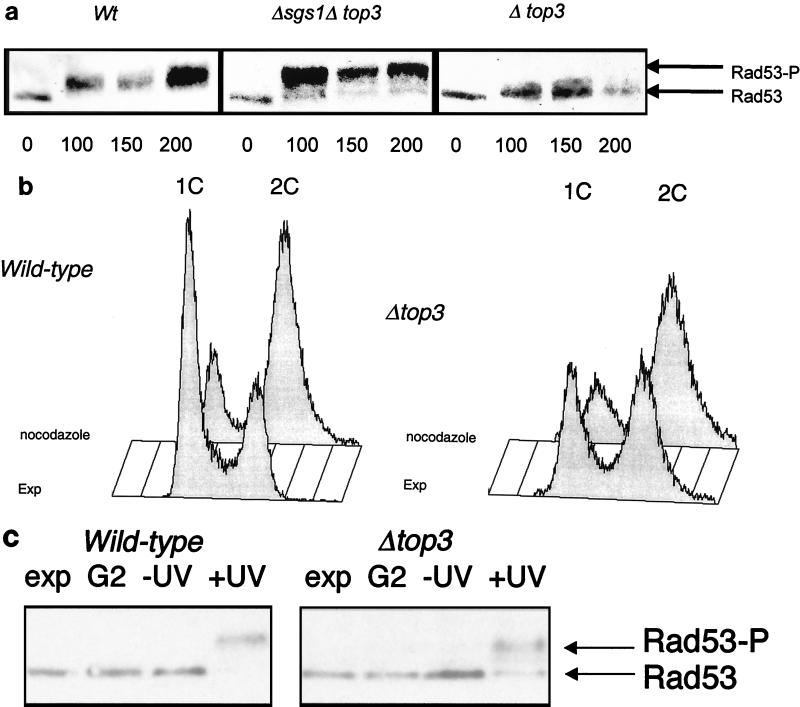
FIG. 4.
Rad53 phosphorylation in top3Δ mutants. (a) Wild-type (Wt), top3, and top3 sgs1 cells were grown in YPD medium to early log phase and then arrested in G1 with α-factor for 150 min. The cells were then released into YPD medium containing 0.03% MMS. Protein extracts derived from cells removed at timed intervals (as indicated in minutes below the lanes) were then separated by SDS-PAGE, transferred to nylon, and immunoblotted with an anti-Rad53 antibody. A representative Western blot for extracts from wild-type, top3 sgs1, and top3 cells is shown. The positions of unphosphorylated Rad53 and phosphorylated Rad53 (Rad53-P) are shown on the right. (b) Flow cytometric analysis to confirm the arrest of wild-type (left) and top3 (right) strains after exposure to nocodazole for 90 min. A population of exponentially growing cells (EXP) is shown in each case for comparison. Peaks representing cells with a 1C and a 2C DNA content are indicated. (c) Phosphorylation of Rad53 in wild-type and top3 strains (as indicated above the lanes) following UV irradiation in G2/M. Lanes: Exp, unirradiated exponentially growing culture; G2, nocodazole-arrested culture; −UV, cells released from nocodazole arrest, mock irradiated, and incubated for 90 min; +UV, cells released from nocodazole arrest, UV irradiated, and incubated for 90 min. The positions of the nonphosphorylated and phosphorylated bands of Rad53 protein, detected by Western blotting as for panel a, are indicated on the right.