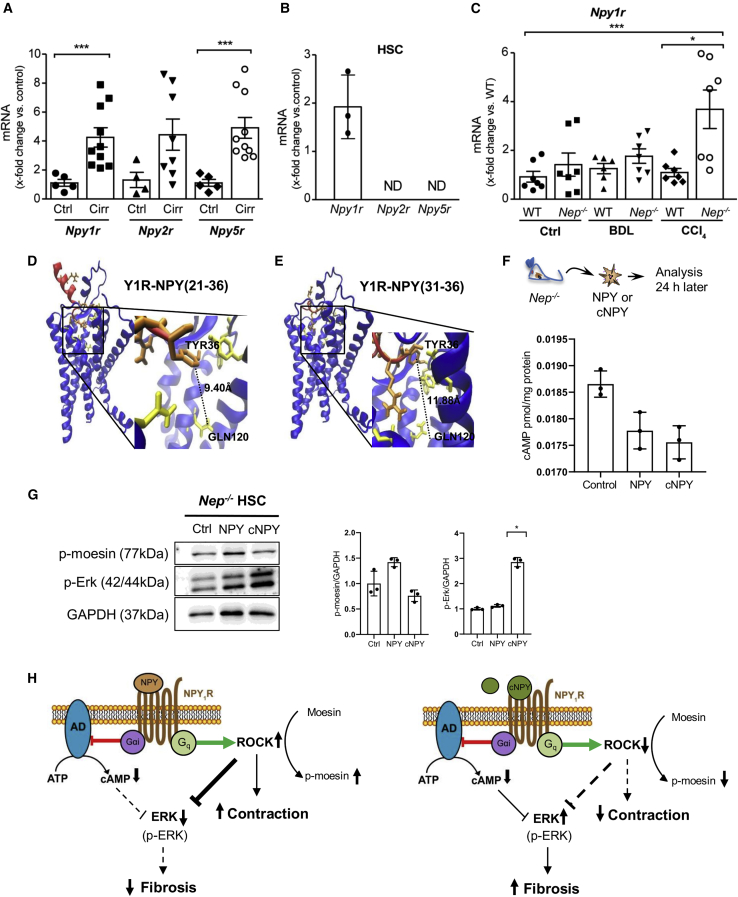
Figure 4.
Cleavage of NPY by NEP shifts the physiological response of Y1R in HSC
(A) Hepatic Npy1r, -2r, and -5r mRNA levels in healthy and cirrhotic patients. Results are expressed as mean ± SEM; n = 5–10 per group, ∗∗∗p < 0.001 for cirrhotic vs. healthy patients.
(B) Npy1r, -2r, and -5r mRNA levels in WT mouse HSCs.
(C) Hepatic Npy1r mRNA levels in CCl4- and BDL-treated Nep−/− and WT mice. Results are expressed as mean ± SEM; ∗p < 0.05 for CCl4 vs. control and ⋅p < 0.05 for Nep−/− vs. WT mice.
(D and E) Structural models of the Y1R-NPY (21–36) and Y1R-NPY (31–36) complexes. Y1R is represented in blue and its interacting residues in yellow. The NPY fragments are shown in red, while the NPY aa residues are colored orange. The distances between residues are indicated by dashed lines.
(F) Primary Nep−/− HSCs were isolated and treated with NPY and cNPY. After 24 h, cAMP concentrations were analyzed in response to cNPY and full-length NPY. Results are expressed as pmol cAMP per mg of total protein. Results are expressed as mean ± SEM; n = 3–4 per group, ∗p < 0.05 for cNPY-treated vs. full-length NPY-treated Nep−/− HSC.
(G) Western blot analysis from Nep−/− HSC control, NPYFL, and cNPY and quantification of p-ERK and p-moesin protein expression using GAPDH as a loading control.
(H) Diagram of NPYFL (left) and cNPY (right) showing the activation of the receptor (NPY1R). The binding of NPY and cNPY activates the inhibitory effect of G protein and inhibits the enzyme adenylate cyclase (AD), which in turn decreases the production of cAMP. Downstream pathways regulated by cAMP, p-ERK, and p-moesin are downregulated or upregulated, depending on the presence of NPYFL (left) or cNPY (right). NPYFL (left) decreases fibrosis and increases contraction, and cNPY (right) increases fibrosis and decreases contraction in the absence of NEP.