This randomized clinical trial compares outcomes of invasive vs conservative strategies in older adults with frailty and non–ST-segment elevation acute myocardial infarction at 1 year.
Key Points
Question
Does a routine invasive strategy improve outcomes in older adults with frailty and non–ST-segment elevation acute myocardial infarction (NSTEMI)?
Findings
In this prematurely terminated randomized clinical trial of 167 older adult patients with frailty and NSTEMI, a routine invasive vs conservative strategy did not significantly increase the number of days alive and out of the hospital from discharge to 1 year. Although the study is small, there is no hint that increasing the number of patients would have confirmed the invasive management superiority hypothesis, as shown in previous studies in older patients where frailty status had not been considered.
Meaning
In this randomized clinical trial including frail older patients with NSTEMI, a routine invasive strategy did not increase the number of days alive out of hospital during the first year; results fuel the development of a larger trial with the opposite hypothesis—that is, a policy of watchful observation and careful evaluation may be of choice for frail older patients with NSTEMI.
Abstract
Importance
To our knowledge, no randomized clinical trial has compared the invasive and conservative strategies in frail, older patients with non–ST-segment elevation acute myocardial infarction (NSTEMI).
Objective
To compare outcomes of invasive and conservative strategies in frail, older patients with NSTEMI at 1 year.
Design, Setting, and Participants
This multicenter randomized clinical trial was conducted at 13 Spanish hospitals between July 7, 2017, and January 9, 2021, and included 167 older adult (≥70 years) patients with frailty (Clinical Frailty Scale score ≥4) and NSTEMI. Data analysis was performed from April 2022 to June 2022.
Interventions
Patients were randomized to routine invasive (coronary angiography and revascularization if feasible; n = 84) or conservative (medical treatment with coronary angiography for recurrent ischemia; n = 83) strategy.
Main Outcomes and Measures
The primary end point was the number of days alive and out of the hospital (DAOH) from discharge to 1 year. The coprimary end point was the composite of cardiac death, reinfarction, or postdischarge revascularization.
Results
The study was prematurely stopped due to the COVID-19 pandemic when 95% of the calculated sample size had been enrolled. Among the 167 patients included, the mean (SD) age was 86 (5) years, and mean (SD) Clinical Frailty Scale score was 5 (1). While not statistically different, DAOH were about 1 month (28 days; 95% CI, −7 to 62) greater for patients managed conservatively (312 days; 95% CI, 289 to 335) vs patients managed invasively (284 days; 95% CI, 255 to 311; P = .12). A sensitivity analysis stratified by sex did not show differences. In addition, we found no differences in all-cause mortality (hazard ratio, 1.45; 95% CI, 0.74-2.85; P = .28). There was a 28-day shorter survival in the invasive vs conservatively managed group (95% CI, −63 to 7 days; restricted mean survival time analysis). Noncardiac reasons accounted for 56% of the readmissions. There were no differences in the number of readmissions or days spent in the hospital after discharge between groups. Neither were there differences in the coprimary end point of ischemic cardiac events (subdistribution hazard ratio, 0.92; 95% CI, 0.54-1.57; P = .78).
Conclusions and Relevance
In this randomized clinical trial of NSTEMI in frail older patients, there was no benefit to a routine invasive strategy in DAOH during the first year. Based on these findings, a policy of medical management and watchful observation is recommended for older patients with frailty and NSTEMI.
Trial Registration
ClinicalTrials.gov Identifier: NCT03208153
Introduction
Frailty has a detrimental impact on prognosis in older adults with non–ST-segment elevation acute myocardial infarction (NSTEMI).1,2,3,4,5,6,7,8 The optimal management of frail patients with NSTEMI, ie, invasive or conservative strategies, remains unknown. Previous reports regarding frailty and NSTEMI management derive from registries with contradictory results.9,10,11 So far, to our knowledge, no clinical trials have targeted frail patients.
The European guidelines recommend the same diagnostic and interventional strategies for older and younger patients.12 However, geriatric conditions, such as frailty, play an essential role in prognosis. Indeed, the European guidelines recognize the absence of robust data on the management of frail patients with NSTEMI and recommend that the potential advantages of treatments must be balanced against the risk of harm. In patients with NSTEMI, frailty is associated with a worse prognosis and a greater risk of complications related to medication or cardiac interventions.1
Although previous studies in older patients with NSTEMI demonstrated the superiority of invasive management, frailty was not evaluated and was probably underrepresented.13,14,15 This trial aimed to test the superiority of a routine invasive compared with a conservative strategy in frail older patients with NSTEMI.
Methods
Study Design and Participants
The MOSCA-FRAIL clinical trial was a multicenter, prospective, randomized, open-label trial that was conducted in older adult patients with frailty and NSTEMI (NCT03208153) (trial protocol in Supplement 1). The inclusion criteria were (1) NSTEMI, defined by symptoms consistent with acute myocardial ischemia, absence of persistent ST-segment elevation, and troponin elevation (according to the local laboratory troponin assay); (2) age 70 years or older; and (3) frailty defined by 4 points or greater on the Clinical Frailty Scale.16 The Clinical Frailty Scale classifies frailty into 9 categories of progressive impairment based on clinical judgment. The definition of each category is shown in eFigure 1 in Supplement 2. Exclusion criteria were prior known nonrevascularizable coronary artery disease, significant concomitant nonischemic heart disease, inability to understand/sign informed consent (patients or relatives), and life expectancy less than 12 months. In addition to the defined inclusion and exclusion criteria, the attending cardiologist should believe that the participation of the patient in the study was reasonable. Reasons for considering participation inappropriate were either the consideration by the attending cardiologist of invasive management being mandatory because of severe clinical instability at admission (recurrent chest pain and/or dynamic ischemic electrocardiogram changes) or any factor making invasive management not an option.
The trial was an investigator-driven initiative under the auspices of the Spanish Society of Cardiology and the official working groups of Interventional Cardiology and Geriatric Cardiology. A total of 13 centers participated in the study (eAppendix 2 in Supplement 2). All centers received the approval of their Medical Ethics Committee, and all patients provided written informed consent. A description of the committees can be found in the eAppendix 1 in Supplement 2. The study design has been published elsewhere.17 This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Randomization and Management
Participants were randomized within 48 hours of admission to 1 of the 2 management strategies: (1) routine invasive strategy, consisting of coronary angiography within 72 hours of admission with coronary revascularization if deemed appropriate, or (2) conservative strategy, consisting of medical therapy only, although cardiac catheterization was allowed in the case of recurrent ischemia during the index hospitalization. Patients were assigned to both treatment groups using a computer-generated randomization scheme to allocate participants in a 1:1 ratio. The randomization was performed via a website where the process was concealed from the researchers until the interventions were assigned.
Medical treatment was optimized according to the clinical practice guidelines recommendations for all patients.12 The standard period of dual antiplatelet therapy was 1 year in both study arms by default. However, in patients at high bleeding risk (in the judgment of the treating physician) or in need of oral anticoagulation therapy, 1 antiplatelet drug could be withdrawn after the first month. When the percutaneous coronary intervention was performed, the type of stent implanted was left to the judgment of the treating cardiologist, although drug-eluting stents were recommended.
A systematic and comprehensive geriatric evaluation was performed during hospitalization in all patients, assessing the status before admission as follows: (1) In addition to the Clinical Frailty Scale, frailty was assessed with the FRAIL scale, which includes evaluation of fatigue (time feeling tired during the past 4 weeks), resistance (ability to climb stairs), ambulation (ability to walk a certain number of meters), concomitant diseases (number of illnesses), and weight loss (self-reported loss of weight within the past 12 months).18 FRAIL scale scores range from 0 to 5 points. (2) Comorbidity conditions were evaluated with the Charlson index.19 (3) Physical independence was evaluated with the Barthel index.20 (4) Cognitive function was measured with the Pfeiffer test.21
Outcomes
The primary end point was the number of days alive and out of the hospital (DAOH) between discharge from the index hospitalization to 1 year, which was considered a meaningful outcome for these patients. This end point is an alternative metric that encompasses both mortality and all hospitalizations, and it might best reflect the success of the management strategy.22 The coprimary end point was the composite of major ischemic cardiac events, including cardiac death, reinfarction, or postdischarge revascularization. Local investigators reported all events and classified all readmissions by cause, but final adjudications were done centrally after reviewing the anonymized source documents. Cardiac death was defined as any death due to cardiac causes. Unwitnessed death and death of unknown cause were considered cardiac death. Readmissions due to cardiac diagnoses were reinfarction (chest pain with troponin elevation), unstable angina (readmission for ischemic chest pain with normal troponin levels), coronary revascularization not related to readmissions for myocardial infarction or unstable angina, acute heart failure, and other cardiac reasons. Noncardiac diagnoses for readmission included stroke, bleeding, and other noncardiac reasons (pulmonary, abdominal, neurologic, diabetes decompensation, infections, neoplasia, peripheral artery disease, falls, urinary, and others). Days during each readmission were counted. Follow-up was carried out via clinical visit, electronic medical record review, and/or telephone contacts at 6 months and 1 year.
Sample Size
The overall sample size for the study was calculated for the primary end point at 1 year, under the hypothesis that invasive management was superior. There was no available information to estimate this end point in frail patients with NSTEMI. In a previous study in older adult patients with NSTEMI and comorbidities, patients who underwent a conservative strategy remained alive out of the hospital for a mean (SD) of 273 (123) days during the first year after discharge.23 Assuming that frail patients might have a reasonably similar profile and considering an increase of 20% in the proportion of DAOH, that is, 55 days with an invasive strategy (superiority assumption), we estimated a sample size of 176 patients (88 per arm) with an estimated power of 80%, 2-sided α level of .05, and 10% rate of loss to follow-up.
Statistical Analysis
All statistical comparisons were made under the intention-to-treat principle. Results are presented as frequencies or mean (SD), as appropriate. Between-group comparisons were performed using the t test or Fisher exact test. We used standardized differences to evaluate how well matched the baseline characteristics resulted from the randomization of the 2 treatment groups. A value of 0.25 or less was considered a good match.24
The primary end point (DAOH) was compared as a continuous variable between the 2 groups using the analysis of variance test. In addition, the effect of treatment on the primary end point was determined by using mixed regression analysis, with the study center included as a random effect. Results were expressed as means and predicted means (least-square means) with 95% CIs.
Administrative censoring was applied at 1 year after randomization. Thus, patients were censored at the time of death (occurring within 1 year) or the end of the study. The effect of the invasive strategy on all-cause mortality was depicted by a Kaplan-Meier curve and assessed by a Cox regression model. The hazard ratio and 95% CI were calculated. The randomized strategy met the proportionality assumption. We also tested for the difference in survival between the 2 strategies using restricted mean survival analysis. A prespecified subgroup analysis according to comorbidities using the Charlson Index (0; 1-2; 3-4; ≥5 points) was done.
The coprimary composite end point was analyzed using competing risk event analysis. Noncardiac death was accounted for as a competing event. The subdistribution hazard ratio and 95% CIs were calculated. Because of the multicenter nature of the data, all time-to-event analyses included the study center as a stratification variable.
Analysis of rates of recurrent events (as counts) was also performed using the Poisson regression model for bivariate count outcomes. Here, the aim was to test the effect of treatment on the rate of the entire outcome history while adjusting the estimates for informative censoring due to death as a terminal event. Estimates were expressed as incidence rate ratios with 95% CIs.
A 2-sided P value of less than .05 was considered to be statistically significant. All analyses were performed using Stata, version 16.1 (StataCorp).
Results
Patient Population and In-Hospital Management
Between July 7, 2017, and January 9, 2021, 169 patients were initially recruited, of whom 1 withdrew consent, and 1 was excluded after randomization because of inclusion criteria violation. Therefore, the study population consisted of 167 patients, 84 allocated to the invasive group and 83 to the conservative group. The patient distribution across centers and the CONSORT flow diagram are shown in eAppendix 2 and eFigure 2 in Supplement 2, respectively. The rate of enrollment was severely reduced in the last part of the trial due to the COVID-19 pandemic, which led to early trial discontinuation when 95% of the calculated sample size had been enrolled (167 of 176 patients); however, all patients were followed up for 1 year.
Baseline characteristics were well balanced between groups, except for a higher proportion of males (47 [57%] vs 32 [38%]; standardized mean difference = 0.401), previous myocardial infarction (32 [39%] vs 19 [23%]; standardized mean difference = 0.349) and previous percutaneous coronary revascularization (33 [40%] vs 19 [23%]; standardized mean difference = 0.374) in the conservative group vs the invasive group, respectively (Table 1). The mean (SD) age was 86 (5) years. There were no differences in the hemodynamic status at admission, electrocardiogram findings, basic blood test results, or geriatric conditions (Table 1). The mean (SD) value of the Clinical Frailty Scales was 5.1 (0.8).
Table 1. Baseline Patient Characteristics (n = 167).
| Characteristic | Patients, No. (%) | Standardized difference | |
|---|---|---|---|
| Invasive (n = 84) | Conservative (n = 83) | ||
| Demographic data | |||
| Age, mean (SD), y | 86 (5) | 85 (5) | .218 |
| Sex | |||
| Female | 52 (62) | 36 (43) | .401 |
| Male | 32 (38) | 47 (57) | |
| Diabetes | 50 (60) | 43 (52) | .155 |
| Insulin treatment | 19 (23) | 19 (23) | .006 |
| Hypertension | 77 (92) | 76 (92) | .004 |
| Hypercholesterolemia | 63 (75) | 65 (78) | .078 |
| Current smoker | 3 (4) | 2 (2) | .230 |
| Peripheral artery disease | 9 (11) | 9 (11) | .004 |
| Chronic kidney disease | 39 (46) | 35 (42) | .085 |
| Dialysis | 3 (4) | 2 (2.4) | .068 |
| Prior myocardial infarction | 19 (23) | 32 (39) | .349 |
| Prior PCI | 19 (23) | 33 (40) | .374 |
| Prior CABG | 5 (6) | 11 (13) | .248 |
| Prior history of atrial fibrillation | 26 (31) | 19 (23) | .181 |
| Prior stroke | 13 (16) | 17 (21) | .203 |
| Prior admission for heart failure | 13 (16) | 16 (19) | .100 |
| Hemodynamic data | |||
| Blood pressure, mm Hg | |||
| Systolic | 139 (27) | 141 (23) | .060 |
| Diastolic | 69 (16) | 70 (12) | .105 |
| Heart rate, beats/min | 77 (15) | 79 (21) | .095 |
| Killip class | |||
| I | 67 (80) | 61 (74) | .005 |
| II | 11 (13) | 19 (23) | |
| III | 6 (7) | 3 (4) | |
| ECG | |||
| Normal ECG | 26 (31) | 27 (33) | .127 |
| ST-segment depression | 34 (41) | 25 (30) | .187 |
| T-wave inversion | 9 (11) | 16 (19) | .043 |
| Left bundle-branch block | 10 (12) | 10 (12) | .040 |
| Pacemaker | 5 (6) | 5 (6) | .003 |
| Blood test | |||
| Hemoglobin, mean (SD), g/dL | 12.4 (2) | 12.4 (2) | .008 |
| Creatinine, mean (SD), mg/dL | 1.4 (1) | 1.3 (1) | .056 |
| Geriatric conditions | |||
| Clinical Frailty Scale score | |||
| 4 | 23 (27) | 20 (24) | .073 |
| 5 | 32 (38) | 40 (48) | |
| 6 | 26 (31) | 22 (27) | |
| 7 | 3 (4) | 1 (1) | |
| Frail scale, mean (SD), points | 2.5 (1) | 2.5 (1) | .015 |
| Charlson index, mean (SD), points | 2.5 (2) | 3.0 (2) | .234 |
| Barthel index, mean (SD), points | 75 (23) | 75 (20) | .001 |
| Pfeiffer test, mean (SD), errors | 1.8 (2) | 2.0 (2) | .140 |
Abbreviations: CABG, coronary artery bypass graft; ECG, electrocardiogram; PCI, percutaneous coronary intervention.
Table 2 shows the hospital management during the index episode. Nearly all patients (82 of 84 [98%]) in the invasive group underwent coronary angiography, resulting in a 60% rate of initial revascularization. Only 2 patients underwent coronary surgery; as a result, revascularization was complete in only 27 of 84 (32%) patients. A total of 9 (11%) patients in the conservative group crossed over to invasive management because of recurrent ischemia (as prespecified in the study protocol), leading to a 9.6% revascularization rate. Radial access and drug-eluting stents were mostly used. There were no differences in mean (SD) predischarge left ventricular ejection fraction evaluated by echocardiography (invasive, 56% [12%]; conservative, 54% [12%]; P = .28). Likewise, the medical treatment prescribed at discharge was similar in both groups, with the exception of β-blockers, which were prescribed more often in the conservative group (65 [83%] vs 56 [69%]; P = .04) (Table 2).
Table 2. Hospital Management.
| Variable | Patients, No. (%) | P value | |
|---|---|---|---|
| Invasive (n = 84) | Conservative (n = 83) | ||
| Coronary angiography | 82 (98) | 9 (11) | <.001 |
| Radial accessa | 69 (84) | 9 (100) | .35 |
| No. of vessels diseaseda | |||
| 0 | 16 (20) | 1 (11) | NA |
| 1 | 25 (30) | 2 (22) | NA |
| 2 | 12 (15) | 2 (22) | NA |
| 3 | 29 (35) | 4 (45) | NA |
| Left main diseasea | 13 (16) | 1 (11) | .67 |
| Coronary revascularization | 50 (60) | 8 (10) | <.001 |
| PCI | 48 (57) | 8 (10) | <.001 |
| Drug-eluting stentsb | 46 (96) | 6 (75) | .06 |
| CABG | 2 (2) | 0 | .16 |
| Complete revascularization | 27 (32) | 4 (5) | <.001 |
| Length of stay, median (IQR), d | 8.9 (12) | 9.6 (11) | .67 |
| Medical treatment at discharge | |||
| Aspirin | 70 (86) | 72 (92) | .23 |
| Clopidogrel | 58 (72) | 54 (69) | .74 |
| Ticagrelor | 7 (9) | 8 (10) | .73 |
| Prasugrel | 0 | 0 | NA |
| Dual antiplatelet therapy | 57 (70) | 57 (73) | .73 |
| NOACs | 14 (17) | 13 (17) | .92 |
| Antivitamin K | 8 (10) | 11 (14) | .41 |
| Triple antithrombotic therapy | 8 (10) | 9 (12) | .80 |
| Statins | 75 (93) | 74 (95) | .55 |
| β-Blockers | 56 (69) | 65 (83) | .04 |
| ACE inhibitors/ARB | 47 (58) | 51 (65) | .42 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CABG, coronary artery bypass graft; NA, not applicable; NOACs, novel oral anticoagulants; PCI, percutaneous coronary intervention.
Percentage of the coronary angiographies.
Percentage of PCIs.
There were no losses to follow-up at 1 year. A total of 38 patients died (7 of them during the index hospitalization).
Days Alive and Out of the Hospital
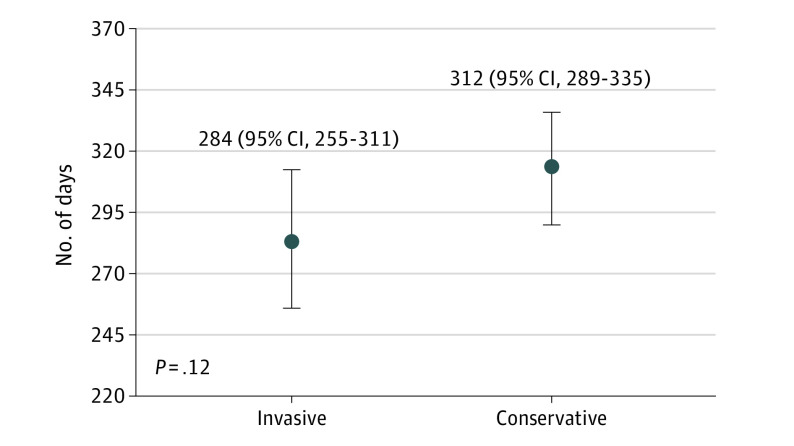
Figure 1 shows the results for the primary end point. There were no missing outcomes. While not statistically different, DAOH was 28 days (95% CI, −7 to 62) less in patients invasively managed compared with those conservatively managed (284 days; 95% CI, 255 to 311, vs 312 days; 95% CI, 289 to 335; P = .12; Figure 1). The same was confirmed after accounting for the center effect using mixed regression analysis. Since there was a sex difference between groups, we conducted a sensitivity analysis stratified by sex, which did not show differences. Neither were differences observed across the Charlson index. The trend in all-cause mortality was in the same direction, ie, we found no differences in all-cause mortality (hazard ratio, 1.45; 95% CI, 0.74-2.85; P = .28; Figure 2), which translated into a 28-day shorter survival during the first year in the invasive vs the conservatively managed group (95% CI, −63 to 7 days) (Figure 2).
Figure 1. Results for the Primary End Point.
Number of days alive and out of the hospital in both study groups.
Figure 2. Results for All-Cause Mortality.
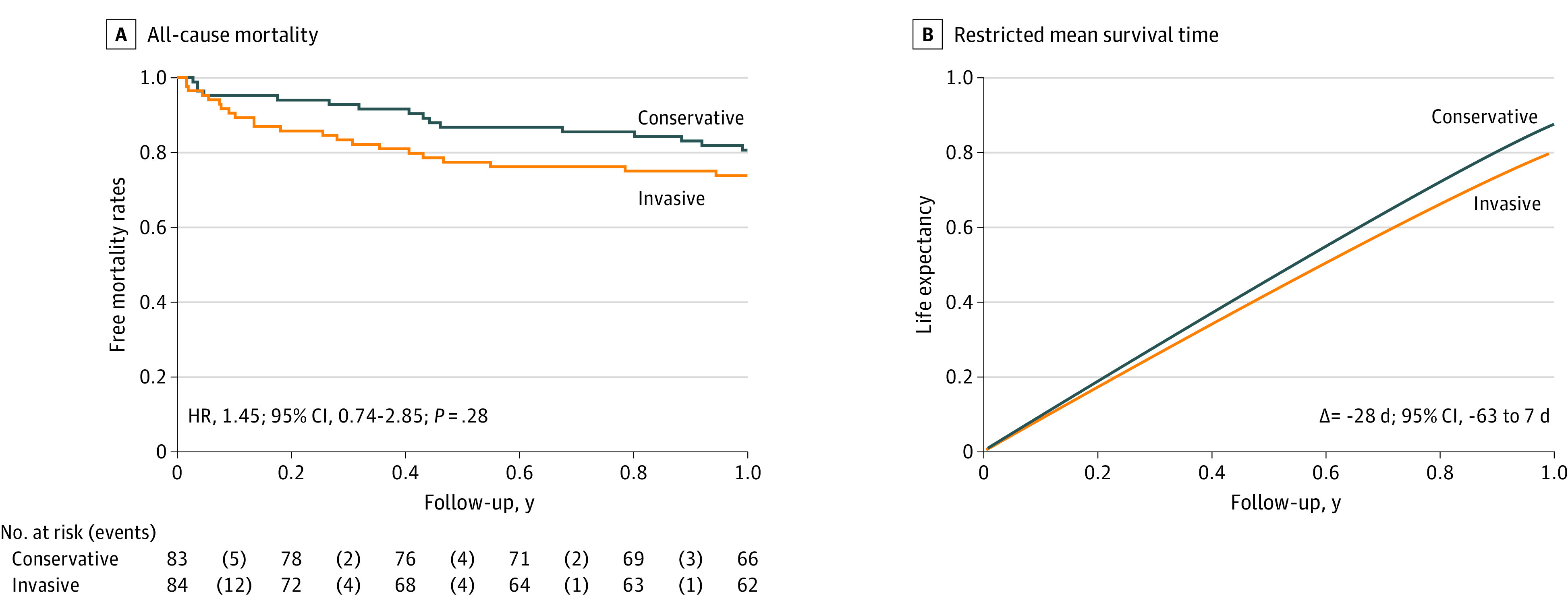
A, Kaplan-Meier curves for all-cause mortality. B, Restricted mean survival time analysis. HR indicates hazard ratio.
During the year after discharge, 86 (51%) patients were readmitted, with a total of 153 hospital stays (including recurrent stays) for the following reasons: acute myocardial infarction (n = 25), unstable angina (n = 4), heart failure (n = 27), planned coronary revascularization (n = 3), other cardiac reasons (n = 8), stroke (n = 8), bleeding (n = 11), and other noncardiac reasons (n = 67). Overall, 86 (56%) readmissions were due to noncardiac reasons. There were no differences in the number of readmissions between groups (invasive: 0.83 readmissions; 95% CI, 0.62-1.04; conservative: 1.0 readmissions; 95% CI, 0.67-1.33; P = .40) or number of days spent in the hospital (invasive: 8 days; 95% CI, 5-11; conservative: 10 days; 95% CI, 6-14; P = .39) (eFigure 3 in Supplement 2).
We analyzed the association of invasive management with all events requiring rehospitalization classified according to their diagnosis while adjusting for informative dropout due to death (eTable in Supplement 2). The only difference was in bleeding. Indeed, the invasive strategy was significantly associated with a higher risk of bleeding requiring hospitalization (incidence rate ratio, 14.9; 95% CI, 1.7-129; P = .02), including 4 deaths related to bleeding. In the conservative group, 1 patient was hospitalized for bleeding 267 days after discharge. Conversely, 8 patients had bleeding episodes in the invasive group (1 had 3 rehospitalizations). The mean time for the first bleeding admission in the invasive group was 159 days (95% CI, 16-344 days).
Coprimary End Point
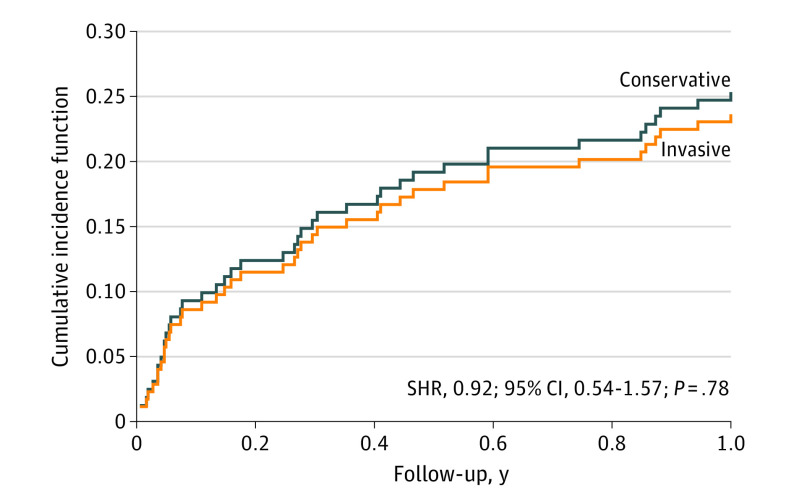
There were no differences between conservative and invasive strategies in the combined coprimary end point: subdistribution hazard ratio, 0.92; 95% CI, 0.54-1.57; P = .78. The analysis was adjusted for noncardiac death as a competing event. Figure 3 displays the curves favoring invasive management, but the trend was not statistically significant. The analysis stratified by sex did not lead to significant differences.
Figure 3. Cumulative Incidence Function Curves for the Coprimary End Point.
Comparison of the composite end point, including cardiac death, reinfarction, and revascularization, between the study groups. Analysis adjusted for noncardiac death as a competing event. SHR indicates subdistribution hazard ratio.
Discussion
To our knowledge, this is the first trial comparing an invasive and a conservative strategy specifically conducted in frail older patients with NSTEMI, an important group among patients with NSTEMI for which there is no available evidence. Our study showed no significant differences between the 2 strategies in the number of DAOH. The trial is small and with limited statistical power. However, there is no hint that increasing the number of patients would have confirmed the hypothesis of invasive management superiority, as had been shown in previous studies in older patients where frailty status had not been considered.13,14,15 The discordant results of our trial compared with other trials on older adult patients may be mainly based on the unique population involved and the novelty of the end point. On the other hand, 2 facts might have also influenced the lack of positive results in the expected direction, ie, favoring the invasive strategy. First, the most unstable patients at admission were not included; second, a crossover was allowed in the conservative arm in the case of an early unfavorable clinical course, although the crossover rate was modest (11%). Our findings should be interpreted with caution but may fuel the development of a larger trial with the opposite hypothesis—that is, a policy of watchful observation and careful evaluation rather than routine invasive management may be as effective for frail patients with NSTEMI.
The advanced age of the current study population (mean [SD], 86 [5] years) is worth noting. The After Eighty trial compared invasive and conservative management in patients older than 80 years (mean age, 85 years).14 Frailty was not assessed, but the long recruiting period and reasons for excluded patients suggest that it was underrepresented. On the other hand, no patient in the conservative arm underwent coronary angiography, regardless of the clinical circumstances. These facts contributed to the results largely favoring invasive management. However, there were no differences in mortality or quality of life.25 A registry including patients older than 80 years and comparing invasive and conservative management using propensity score techniques also supports the superiority of invasive management.15 Still, frailty or other geriatric conditions were not considered, and it was not a randomized trial. The first MOSCA trial included adult patients with NSTEMI and at least 2 comorbidities among 6 screened.23 There were no differences in outcomes between the invasive and conservative approaches, though the study was unpowered. In line with these results, a large registry including over 7000 patients older than 70 years with NSTEMI showed that comorbidities attenuated the potential benefit of in-hospital revascularization for 1-year mortality.26 In a registry of nonagenarians, the percutaneous coronary intervention improved outcomes, but mainly in patients without disabilities.27
To our knowledge, no randomized clinical trial has compared the invasive and conservative strategies in frail older patients with NSTEMI. A small registry of patients with NSTEMI found that in-hospital revascularization improved outcomes in frail patients compared with their nonfrail counterparts.9 However, other registries reported the opposite.10,11 Confounding factors inherent to registries limit their conclusions. Our results could not show the superiority of a routine invasive strategy over a conservative approach in this very high-risk subgroup of patients. The sample size was estimated on the differences in DAOH as a continuous end point. Unfortunately, the sample size appropriate for this end point was insufficient for other outcomes. Consequently, our findings do not contradict the potential benefit of routine invasive management for ischemic cardiac events since the study was not designed for this purpose. On the other hand, surgical revascularization was underused despite complex coronary artery disease due to the surgical risk of the population. This fact led to a low rate of complete revascularization, which could have influenced the results. However, the high rate of noncardiac events during follow-up might neutralize the potential cardiac benefit of invasive management in these patients. Indeed, cardiac ischemic events, which are the most preventable complications by invasive management, were far less frequent than noncardiac events, which is a relevant finding that may help design future trials beyond the traditional cardiac end points selected for patients with NSTEMI. Therefore, frail older patients hospitalized for a specific reason, such as NSTEMI, should not be assessed as a single disease but as a global status that conditions outcomes. The DAOH seems to be an appropriate metric.
Patients invasively managed showed a higher risk of bleeding episodes, though our study lacked statistical power for analyzing individual events; therefore, this might be a chance finding. On the other hand, the antithrombotic treatment prescribed at discharge was the same in both groups as prespecified in the study protocol. However, medication persistence and adherence during follow-up were not collected. It may be speculated that patients in the conservative arm may have had their antithrombotic treatment more often reduced during follow-up since invasive management might be a factor for antithrombotic treatment adherence. The fact that the bleeding episodes occurred late after discharge supports this hypothesis. This finding should be prospectively investigated in future randomized trials regardless of the revascularization strategy used in these patients.
Limitations
Several limitations merit being acknowledged. First, pandemic constraints allowed the recruitment of only 95% of the estimated sample size. Therefore, the study might be underpowered for the primary end point of DAOH. However, the direction of the results does not suggest the superiority of invasive management even if the estimated sample size had been completed. Second, the information about the total number of patients screened for enrollment was not collected. The enrollment was relatively slow, and not all consecutive patients were considered for randomization, which might have resulted in selection bias. Indeed, it should be noted that the attending cardiologist was ultimately responsible for evaluating the opportunity to enroll a patient in the trial. Third, we used the Clinical Frailty Scale, which is a well-validated scale used to quantify the degree of disability from frailty. Clinical conditions on admission preclude using other frailty scales, such as the Fried score. Fourth, the trial has the limitations inherent to an open-label design. Fifth, the COVID-19 pandemic could have played a role in the results of the study. The conservative group may have been more reluctant to come to the hospital during the pandemic. Sixth, the randomization process failed to ensure a perfect balance in baseline characteristics, as may happen with trials of small sample sizes.
Conclusions
In this randomized clinical trial in frail older patients, defined by a Clinical Frailty Scale score of 4 or greater, with NSTEMI and stable clinical conditions at admission, a routine invasive strategy did not increase the number of DAOH 1 year after discharge. Based on this first evidence in this population, a policy of watchful observation could be of choice for patients with frailty and NSTEMI. A larger randomized clinical trial testing the efficacy and safety of a watchful observation strategy in frail patients with NSTEMI is warranted.
Trial Protocol
eAppendix 1. List of the Committees and Members
eAppendix 2. Participant Centres and Number of Patients Recruited
eFigure 1. Clinical Frailty Scale
eFigure 2. CONSORT Flow Diagram
eFigure 3. Readmission After Hospital Discharge
eTable. Invasive Management and Risk of Hospital Stay According to Diagnosis After Discharge of the Index Episode
Data Sharing Statement
References
- 1.Walker DM, Gale CP, Lip G, et al. Editor’s choice—Frailty and the management of patients with acute cardiovascular disease: a position paper from the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care. 2018;7(2):176-193. doi: 10.1177/2048872618758931 [DOI] [PubMed] [Google Scholar]
- 2.Sanchis J, Bonanad C, Ruiz V, et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J. 2014;168(5):784-791. doi: 10.1016/j.ahj.2014.07.022 [DOI] [PubMed] [Google Scholar]
- 3.Sanchis J, Ruiz V, Bonanad C, et al. Prognostic value of geriatric conditions beyond age after acute coronary syndrome. Mayo Clin Proc. 2017;92(6):934-939. doi: 10.1016/j.mayocp.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 4.Sánchez E, Vidán MT, Serra JA, Fernández-Avilés F, Bueno H. Prevalence of geriatric syndromes and impact on clinical and functional outcomes in older patients with acute cardiac diseases. Heart. 2011;97(19):1602-1606. doi: 10.1136/hrt.2011.227504 [DOI] [PubMed] [Google Scholar]
- 5.Alegre O, Formiga F, López-Palop R, et al. ; LONGEVO-SCA registry investigators . An easy assessment of frailty at baseline independently predicts prognosis in very elderly patients with acute coronary syndromes. J Am Med Dir Assoc. 2018;19(4):296-303. doi: 10.1016/j.jamda.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Queraltó O, Formiga F, López-Palop R, et al. ; LONGEVO-SCA registry investigators . FRAIL scale also predicts long-term outcomes in older patients with acute coronary syndromes. J Am Med Dir Assoc. 2020;21(5):683-687.e1. doi: 10.1016/j.jamda.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 7.Ekerstad N, Javadzadeh D, Alexander KP, et al. Clinical Frailty Scale classes are independently associated with 6-month mortality for patients after acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2022;11(2):89-98. doi: 10.1093/ehjacc/zuab114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Díez-Villanueva P, Arizá-Solé A, Vidán MT, et al. Recommendations of the Geriatric Cardiology Section of the Spanish Society of Cardiology for the assessment of frailty in elderly patients with heart disease. Rev Esp Cardiol (Engl Ed). 2019;72(1):63-71. doi: 10.1016/j.rec.2018.06.035 [DOI] [PubMed] [Google Scholar]
- 9.Núñez J, Ruiz V, Bonanad C, et al. Percutaneous coronary intervention and recurrent hospitalizations in elderly patients with non ST-segment acute coronary syndrome: the role of frailty. Int J Cardiol. 2017;228:456-458. doi: 10.1016/j.ijcard.2016.11.151 [DOI] [PubMed] [Google Scholar]
- 10.Llaó I, Ariza-Solé A, Sanchis J, et al. Invasive strategy and frailty in very elderly patients with acute coronary syndromes. EuroIntervention. 2018;14(3):e336-e342. doi: 10.4244/EIJ-D-18-00099 [DOI] [PubMed] [Google Scholar]
- 11.Fishman B, Sharon A, Itelman E, et al. Invasive management in older adults (≥80 Years) with non-ST elevation myocardial infarction. Mayo Clin Proc. 2022;97(7):1247-1256. doi: 10.1016/j.mayocp.2022.03.021 [DOI] [PubMed] [Google Scholar]
- 12.Collet JP, Thiele H, Barbato E, et al. ; ESC Scientific Document Group . 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289-1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 13.Savonitto S, Cavallini C, Petronio AS, et al. ; Italian Elderly ACS Trial Investigators . Early aggressive versus initially conservative treatment in elderly patients with non-ST-segment elevation acute coronary syndrome: a randomized controlled trial. JACC Cardiovasc Interv. 2012;5(9):906-916. doi: 10.1016/j.jcin.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 14.Tegn N, Abdelnoor M, Aaberge L, et al. ; After Eighty study investigators . Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open-label randomised controlled trial. Lancet. 2016;387(10023):1057-1065. doi: 10.1016/S0140-6736(15)01166-6 [DOI] [PubMed] [Google Scholar]
- 15.Kaura A, Sterne JAC, Trickey A, et al. Invasive versus non-invasive management of older patients with non-ST elevation myocardial infarction (SENIOR-NSTEMI): a cohort study based on routine clinical data. Lancet. 2020;396(10251):623-634. doi: 10.1016/S0140-6736(20)30930-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489-495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchis J, Ariza-Solé A, Abu-Assi E, et al. Invasive versus conservative strategy in frail patients with NSTEMI: the MOSCA-FRAIL clinical trial study design. Rev Esp Cardiol (Engl Ed). 2019;72(2):154-159. doi: 10.1016/j.recesp.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 18.Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A. task force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12(1):29-37. doi: 10.1007/BF02982161 [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 20.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61-65. [PubMed] [Google Scholar]
- 21.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433-441. doi: 10.1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- 22.Ariti CA, Cleland JG, Pocock SJ, et al. Days alive and out of hospital and the patient journey in patients with heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J. 2011;162(5):900-906. doi: 10.1016/j.ahj.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 23.Sanchis J, Núñez E, Barrabés JA, et al. Randomized comparison between the invasive and conservative strategies in comorbid elderly patients with non-ST elevation myocardial infarction. Eur J Intern Med. 2016;35:89-94. doi: 10.1016/j.ejim.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 24.Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169-188. doi: 10.1023/A:1020363010465 [DOI] [Google Scholar]
- 25.Tegn N, Abdelnoor M, Aaberge L, et al. ; After Eighty study investigators . Health-related quality of life in older patients with acute coronary syndrome randomised to an invasive or conservative strategy: the After Eighty randomised controlled trial. Age Ageing. 2018;47(1):42-47. doi: 10.1093/ageing/afx121 [DOI] [PubMed] [Google Scholar]
- 26.Sanchis J, García Acuña JM, Raposeiras S, et al. Comorbidity burden and revascularization benefit in elderly patients with acute coronary syndrome. Rev Esp Cardiol (Engl Ed). 2021;74(9):765-772. doi: 10.1016/j.recesp.2020.06.025 [DOI] [PubMed] [Google Scholar]
- 27.Cepas-Guillén PL, Echarte-Morales J, Caldentey G, et al. Outcomes of nonagenarians with acute coronary syndrome. J Am Med Dir Assoc. 2022;23(1):81-86.e4. doi: 10.1016/j.jamda.2021.04.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. List of the Committees and Members
eAppendix 2. Participant Centres and Number of Patients Recruited
eFigure 1. Clinical Frailty Scale
eFigure 2. CONSORT Flow Diagram
eFigure 3. Readmission After Hospital Discharge
eTable. Invasive Management and Risk of Hospital Stay According to Diagnosis After Discharge of the Index Episode
Data Sharing Statement