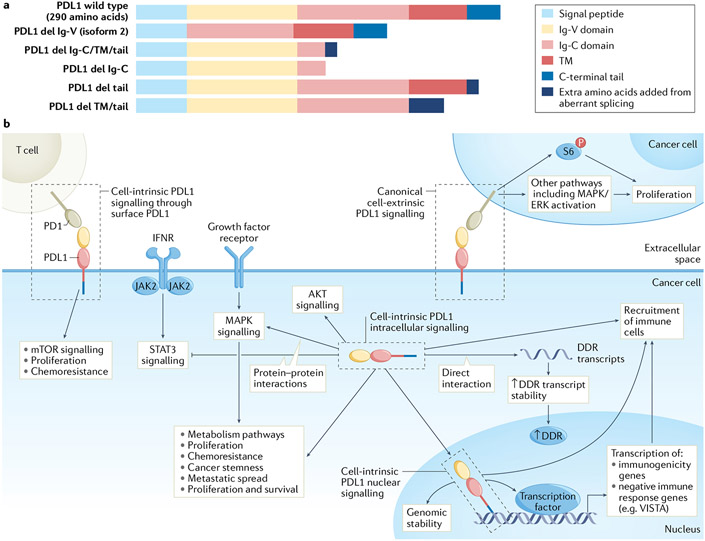
Fig. 1 ∣. Programmed death ligand 1 signalling in cancer cells.
a ∣ Programmed death ligand 1 (PDL1) comprises several domains, the order and presence of which differ between wild-type PDL1 and its isoforms. PDL1 isoforms can be secreted by loss of transmembrane anchoring through splicing or retained in subcellular compartments where they could control cell-intrinsic signals, all requiring further investigation. PDL1 isoforms aside from full-length (isoform 1) PDL1 or its enzymatic cleavage product in vesicles, exosomes or soluble form are not described, b ∣ Surface PDL1 exhibits immunoglobulin-like topology with amino-terminal extracellular structure (yellow and light red rectangles) attached to a transmembrane (TM) domain (dark red rectangle) and a short carboxy-terminal tail (blue rectangle). The immunoglobulin-like extracellular domain interacts with the immunoglobulin-like domain of extracellular programmed death 1 (PD1). Cell-intrinsic full-length intracellular PDL1 (for example, cytoplasmic or nuclear) has same sequence as PDL1 expressed on the cell surface, but its N-terminal domain is likely unfolded and, thus, could interact with proteins distinctly from surface-expressed PDL1, and likely cannot engage PD1 using extracellular topology. Surface PDL1 appears capable of reverse signalling through its cell-intrinsic C-terminal tail (cell-intrinsic PDL1 surface signalling, or reverse PDL1 signalling), requiring additional mechanistic studies to confirm, or could signal to adjacent cancer cells expressing PD1 (cell-extrinsic PDL1 signalling), CD80 or other receptors such as specific integrins. PDL1 in cytosol can directly interact with resident molecules, for example, mRNA and others described in text, or in nucleus to act as a co-transcription factor, or mediate other effects described in text. Tumour surface PDL1 signalling to PD1 on distinct cancer cells (cell-extrinsic PDL1 surface signalling) can promote cancer cell proliferation, for example by increasing phosphorylation of ribosomal S6 protein (p-S6) in PD1-expressing cancer cells among other effects. Cytosolic PDL1 can control mRNA stability to regulate expression of genes involved in the DNA damage response (DDR) or could directly promote major signalling pathways such as mitogen-activated protein kinase (MAPK)/extracellular-signal-related kinase (ERK) or signal transducer and activator of transcription 3 (STAT3) through protein–protein interactions. Nuclear PDL1 exerts transcriptional control of specific target genes involved in tumour immunogenicity or can have non-transcriptional effects on chromosome stability through sister chromatid. These cell-intrinsic PDL1 signals affect antitumour immune responses distinctly from surface PDL1. del, deletion; IFNR, interferon receptor; VISTA, V-type immunoglobulin domain-containing suppressor of T cell activation.