Fig. 3 |. Alternating-access transport mechanism and antifolate-binding model.
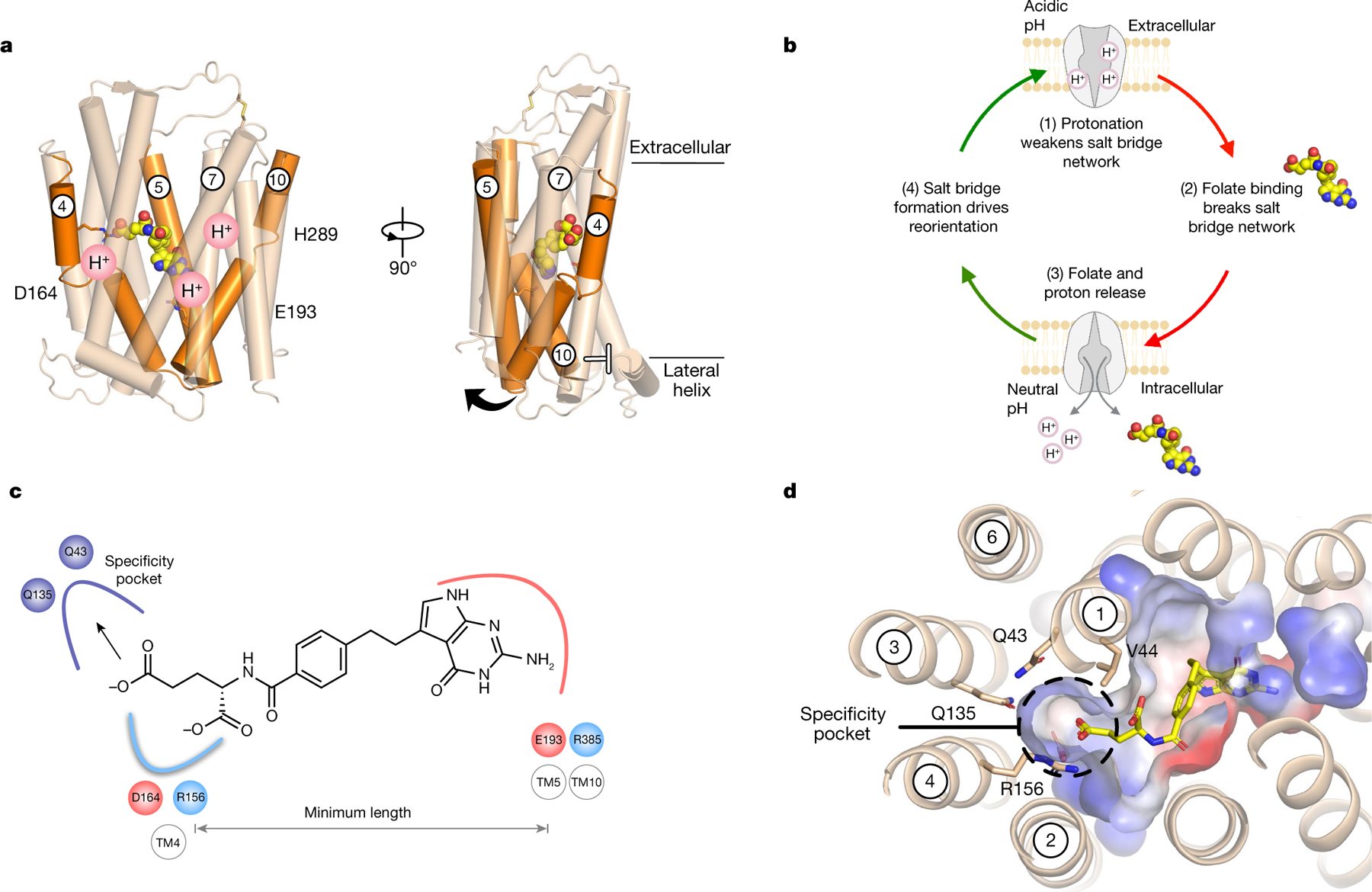
a, Analysis of the gating helices (orange) reveals how proton binding is linked to intracellular gate dynamics. TM4 and TM5 are likely to undergo the largest structural change to release ligand into the cell, whereas proton binding at His289 will facilitate the closure of the extracellular gate mediated by TM7. b, Key steps in the alternating-access model for proton-coupled folate transport. c, Folates or antifolate agents must engage both salt bridges at either end of the binding site to open the intracellular gate, which establishes a minimum length for substrates. d, A polar pocket close to the γ-carboxylate explains how PCFT-specific antifolate drugs are recognized.
