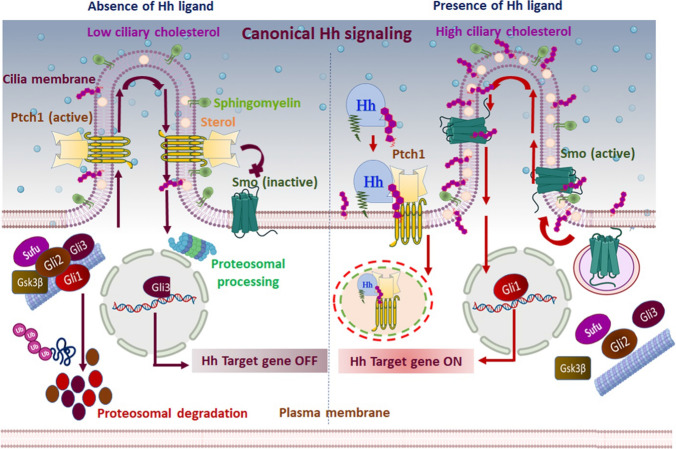
Fig. 2.
Canonical Hh signaling mechanism: the canonical/classical Hh signaling is a ligand-dependent interaction regulated by a bifunctional transcription factor that can activate or repress the transcription of target genes based on nuclear translocation of suppressor/truncated or activator/full-length forms. In the absence of Hh ligand (left panel), Ptch1 accumulates on the primary cilium and inhibits the translocation and functional activation of Smo. Subsequently, Sufu restrains Gli activity, and Ptch1 facilitates the activation of protein kinases (CK1, PKA, and Gsk3β) that induce the phosphorylation of Gli family members. Complete degradation of Gli1, Gli2, and partial cleavage of Gli3 ensue by ubiquitination. The partial cleavage of Gli3 generates the truncated Gli3 that trans-locates to the nucleus and acts as a transcriptional repressor for Hh target genes. Furthermore, Ptch1 inhibits Smo activity by reducing the accessible cholesterol in the ciliary membrane. Without Smo activity, the Gli proteins undergo proteasomal degradation and turn off Hh signal transmission. In the presence of the Hh signal (right panel), Hh binding to Ptch1 inhibits the function of Ptch1 and induces its clearance from the primary cilium via lysosomal degradation of the Hh–Ptch1 complex. Consequentially, inhibition of Smo is lifted, and it relocates to the primary cilium. Activated Smo transmits the Hh signal across the membrane by antagonizing Sufu and protein kinases, ultimately preventing degradation of Gli proteins. Activated Gli protein is translocated into the nucleus and acts as a transcriptional activator for Hh target genes. In addition, the inactivation of Ptch1 raises the accessible cholesterol level in the ciliary membrane, allowing Smo to adopt an active conformation and induce activated Gli. Active Gli trans-locates into the nucleus and ultimately turns on the Hh signal transmission