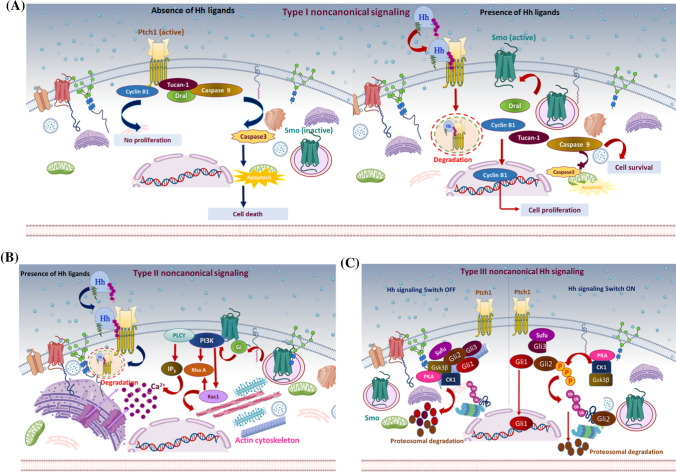
Fig. 3.
Non-canonical hedgehog signaling mechanism: different proposed models of non-canonical Hh signaling regulation. a Type I non-canonical Hh signaling: the C-terminal motif of Ptch1 interacts with cyclin B1 and a proapoptotic complex, including caspase 9, Tucan-1, and Dral. In the absence of the Hh signal (left panel), Ptch1 sequesters cyclin B1 and inhibits its nuclear translocation, inhibiting cell proliferation/survival. Also, cleavage of the C-terminal domain of Ptch1 by caspase 3 exposes the proapoptotic domain and promotes nucleation and activation of caspase 3, eventually leading to apoptosis. In the presence of Hh ligand (right panel), Hh binds Ptch1, and the interaction of Ptch1 with cyclin B1 or the proapoptotic assembly is disrupted, leading to increased cellular proliferation/survival. b Type II non-canonical Hh signaling: In the presence of Hh ligand, Ptch1 becomes degraded, releasing activated Smo to regulate the actin cytoskeleton through a small GTPase in a context-dependent manner. In some cell types, activated Smo releases Ca+2 from the ER via Gi and PLCϒ-dependent generation of IP3 and IP3 channel opening. c Type III non-canonical Hh signaling: In the absence of an Hh signal (left panel), protein kinases PKA, CK1, and Gsk3β phosphorylate Gli family members. This induces ubiquitin-mediated degradation of Gli1 and Gli2. Meanwhile, Gli3 is converted into its truncated repressor form and is translocated into the nucleus. Truncated Gli3 is released from Sufu and blocks Gli1-mediated activation of the target genes. In the presence of the Hh signal (right panel), Gli is released from phosphorylation-mediated proteasomal degradation. Subsequently, Gli1 localizes to the nucleus and activates Hh responsive genes