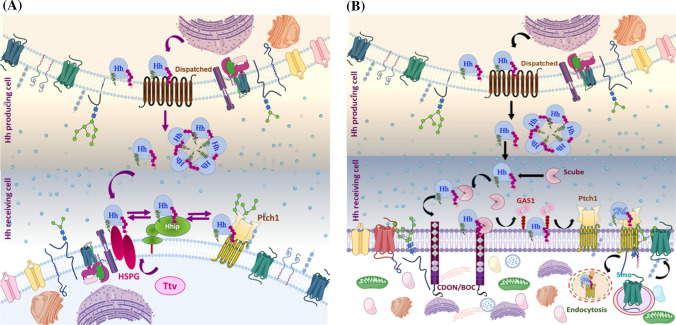
Fig. 5.
Possible scheme for the release and uptake of cholesterol-modified Hh ligand: lipidated Hh associates with the plasma membrane (lipid-raft domain) and binds with the sterol-sensing domain of DispA, and this interaction is essential for Hh release. a Disp and Ttv mediate Hh secretion in Drosophila: once Disp-dependent Hh is released, it moves from the secretory cell to the responding cell by interacting with a Ttv-dependent membrane-tethered proteoglycan HSPG. Further, Ptch1 and Hip limit Hh diffusion by sequestering the Ptch–Hh complex. b Disp and Scube mediate Hh secretion in Vertebrates: From DispA, Hh is transferred to the Scube2 via a hand-off mechanism. Scube binds cholesterol-anchored Hh, producing a highly active and soluble morphogen. Co-receptors, such as CDON/BOC and Gas1, cooperate to move Hh from Scube2 to Ptch1. Briefly, CDON/BOC recruits Scube-bound Hh to the cell surface and facilitates Hh transfer to Gas1, which catalyzes Hh transfer to receptor Ptch1 in responding cells. The binding of Hh to Ptch1 promotes endocytosis of the Ptch–Hh complex, which facilities Smo translocation