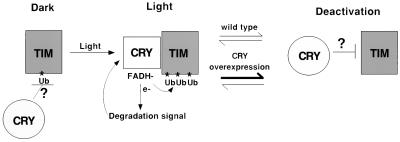
FIG. 8.
Model for the effect of CRY on TIM. The light-induced conformation of CRY promotes TIM ubiquitination, perhaps through direct interaction with TIM. This conformation of CRY also signals to a degradation pathway through a DPI-sensitive electron transport mechanism. As a result, both CRY and TIM are degraded (although TIM degradation is not observed in S2 cells, light-induced TIM degradation in flies is CRY dependent [17] [Fig. 4]). We assume that, like other photoreceptors, CRY is eventually deactivated. Overexpression of CRY may favor its deactivation, resulting in decreased TIM ubiquitination in S2 cells and decreased degradation in flies (Fig. 7). We suggest that the inactive CRY actively inhibits TIM ubiquitination, a mechanism by which CRY could also control TIM ubiquitination in the dark (see Discussion). FADH, reduced flavin adenine dinucleotide.