Abstract
Objectives
We estimated the BNT162b2 vaccine effectiveness (VE) against any (symptomatic or not) SARS-CoV-2 Delta and Omicron infection among adolescents (aged 12-17 years) in Norway from August 2021 to January 2022.
Methods
We used Cox proportional hazard models, where vaccine status was included as a time-varying covariate and models were adjusted for age, sex, comorbidities, residence county, birth country, and living conditions.
Results
The VE against Delta infection peaked at 68% (95% confidence interval [CI]: 64-71%) and 62% (95% CI: 57-66%) in days 21-48 after the first dose among those aged 12-15 years and 16-17 years, respectively. Among those aged 16-17 years who received two doses, the VE against Delta infection peaked at 93% (95% CI: 90-95%) in days 35-62 and decreased to 84% (95% CI: 76-89%) in ≥63 days after vaccination. We did not observe a protective effect against Omicron infection after receiving one dose. Among those aged 16-17 years, the VE against Omicron infection peaked at 53% (95% CI: 43-62%) in 7-34 days after the second dose and decreased to 23% (95% CI: 3-40%) in ≥63 days after vaccination.
Conclusion
We found a reduced protection after two BNT162b2 vaccine doses against any Omicron infection compared to Delta. Effectiveness decreased with time from vaccination for both variants. The impact of vaccination among adolescents on reducing infection and thus transmission is limited during the Omicron dominance.
Keywords: Vaccine effectiveness, BNT162b2, Delta, Omicron, Adolescents, SARS-CoV-2
Introduction
Even though COVID-19 is generally mild in children of all ages [1], [2], [3], strict and widespread infection prevention measures to control transmission in society have disrupted education and social activities [4]. To provide advice for infection control among children and adolescents, including vaccination, it is essential to understand the role of children in transmission and their disease burden, as well as the effect and consequences of the implemented measures.
Overall, vaccines are considered important to fight the spread of the COVID-19 and prevent serious outcomes. BNT162b2 (Comirnaty, Pfizer-BioNTech) and messenger RNA-1273 (Spikevax, Moderna) vaccines have been approved for use in adolescents, based on randomized placebo-controlled trials demonstrating comparable immunogenicity and safety profiles as in young adults [5,6]. In addition, several observational studies have shown that BNT162b2 protects against infection, hospitalization, and/or intensive care unit admissions by the SARS-CoV-2 Delta variant among children and/or adolescents [7], [8], [9], [10], [11], [12], [13], [14]. Evidence on BNT162b2 protection against Omicron variant in children and adolescents is still sparse with few studies suggesting lower vaccine effectiveness (VE) against infection and against COVID-19 hospitalization than Delta [13], [14], [15], [16].
Since August 18, 2021, Norway has recommended two doses of BNT162b2 for those aged 16-17 years, with an extended interval of 8-12 weeks between doses. In addition, since September 2, 2021, those aged 12-15 years were offered a single dose of BNT162b2 and a second dose from the end of January 2022 [17]. From mid-August 2021 (at school re-entry), testing in secondary schools was widespread and lasted until mid-January. All class contacts of cases were encouraged to test, and in September, additional routine biweekly testing in secondary schools was introduced in areas and periods of high incidence. All testing was free of charge and easily accessible to ensure a high uptake. Most adolescents aged 12-17 years would be tested using rapid antigen test, followed by a polymerase chain reaction (PCR) test to confirm if positive. In Norway, the Delta variant was predominant from August to December 2021. The Omicron variant was first detected in adults at the end of November and overtook Delta by the end of the year [18].
Concerns regarding the VE against Omicron variants was raised after a rapid increase in the SARS-CoV-2 incidence in highly vaccinated populations, including among adolescents. In a population-based cohort study, we estimated the VE of BNT162b2 against PCR-confirmed (regardless of symptoms) SARS-CoV-2 Delta or Omicron infection among adolescents (aged 12-17 years) in Norway from August 2021 to January 2022.
Methods
Study design and data sources
We conducted a retrospective population-based cohort study for the period August 25, 2021 to January 16, 2022, including all individuals born from 2004 to 2009 (aged 12-17 years), who were registered as living in Norway with a valid national identity number. Of these 390,992 individuals, we excluded 4374 individuals with less than 19 days between the first and second dose, those with four registered vaccine doses, or who received another vaccine product (not BNT162b2). In addition, we excluded 14,439 individuals with a reported SARS-CoV-2 infection before August 25, 2021. This study period includes the whole period that vaccination was recommended to these two age groups, as well as enhanced and comparable testing strategy.
We obtained data from the Norwegian national preparedness registry for COVID-19 (Beredt C19) [19] that contains individual-level data from the national health and administrative registries, which can be linked using the national identification number. It covers all residents in Norway and includes data on all laboratory-confirmed cases of COVID-19, all hospitalizations, and vaccination (Supplement, part 1). The individual-level data used for this study included age (in years), sex, county of residence (12 levels), household crowding (three levels; yes, no, unknown), dates of vaccination, vaccine type (only BNT162b2 included), underlying comorbidities (three levels: low risk [no underlying comorbidities], medium risk, and high risk comorbidity), date of sampling for reported cases, and information on the SARS-CoV-2 variant if available (Supplement, part 1). We extracted data from the registries on February 18, 2022.
Definitions
SARS-CoV-2 infection
We defined SARS-CoV-2 infection as a positive SARS-CoV-2 PCR test reported to the Norwegian Surveillance System for Communicable Diseases registry. We used the testing date as the time of infection (positive PCR test). Both symptomatic and asymptomatic reported cases have been included because it is not possible to distinguish between these in the Norwegian Surveillance System for Communicable Diseases registry.
SARS-CoV-2 Delta or Omicron variants
Identified using Sanger partial S-gene sequencing or PCR screening targeting specific single nucleotide polymorphisms, insertions, or deletions; the details for laboratory testing for the variants were previously described [20].
Vaccination status
Vaccine status was defined based on number of doses and date of vaccination recorded in the Norwegian Immunisation Registry (SYSVAK). We used 4-week intervals from the time a received dose was considered valid (21 days after vaccination for dose one and 7 days after vaccination for dose two) to assess the waning of VE, while taking into account our sample size, the start of the vaccination of adolescents in Norway, and the allowed follow-up period for the two variants. We defined the categories for vaccine status as
-
1.
Unvaccinated, no dose received
-
2.
One dose, 0-20 days after the first vaccine dose
-
3.
One dose, 21-48 days after the first vaccine dose
-
4.
One dose, 49-76 days after the first vaccine dose
-
5.
One dose, ≥77 days after the first vaccine dose
-
6.
Two doses, 0-6 days after the second vaccine dose
-
7.
Two doses, 7-34 days after the second vaccine dose
-
8.
Two doses, 35-62 days after the second vaccine dose
-
9.
Two doses, ≥63 days after the second vaccine dose
Data analysis
Statistical analysis
We estimated the VE against any SARS-CoV-2 infections (Delta and Omicron infections in separate analyses) using a Cox proportional hazards models with vaccination status as a time-dependent covariate and allowing changes in the baseline hazard over time. In these analyses, we stratified for the available factors associated with the likelihood of being vaccinated and being infected. All our adjusted VE estimates were stratified for sex, country of birth, county of residence, crowding, and underlying comorbidities associated with increased risk of severe COVID-19. VE is defined as 100 × (1 - β), with β being the proportional hazard associated with vaccine status. Further details on the statistical analysis can be found in the Supplement part 2. The statistical significance level was set at 0.05.
More specifically, we estimated the VE among individuals with no previous SARS-CoV-2 infection after receiving one dose for those aged 12-15 years and after one and two doses for those aged 16-17 years against (a) all reported infections from August 25 to November 25, 2021; (b) reported Delta infections from August 25, 2021 to January 16, 2022; and (c) reported Omicron infections from November 26, 2021 to January 16, 2022. Between August 25 to November 25, Delta accounted for nearly 100% of all samples typed, and therefore, we assume that the analyses of all reported cases in this period are Delta infections. The follow-up period ended on January 16 because the comprehensive screening activity in Norway ceased as Omicron reached more than 90% prevalence. We performed additional analyses to evaluate the impact in estimating VE for a specific variant when only including (using data) screened cases because only part of all reported cases is screened for the variant (Supplement part 2).
Statistical analysis was performed in Stata version 16 (Stata Corporation, College Station, TX, USA).
Results
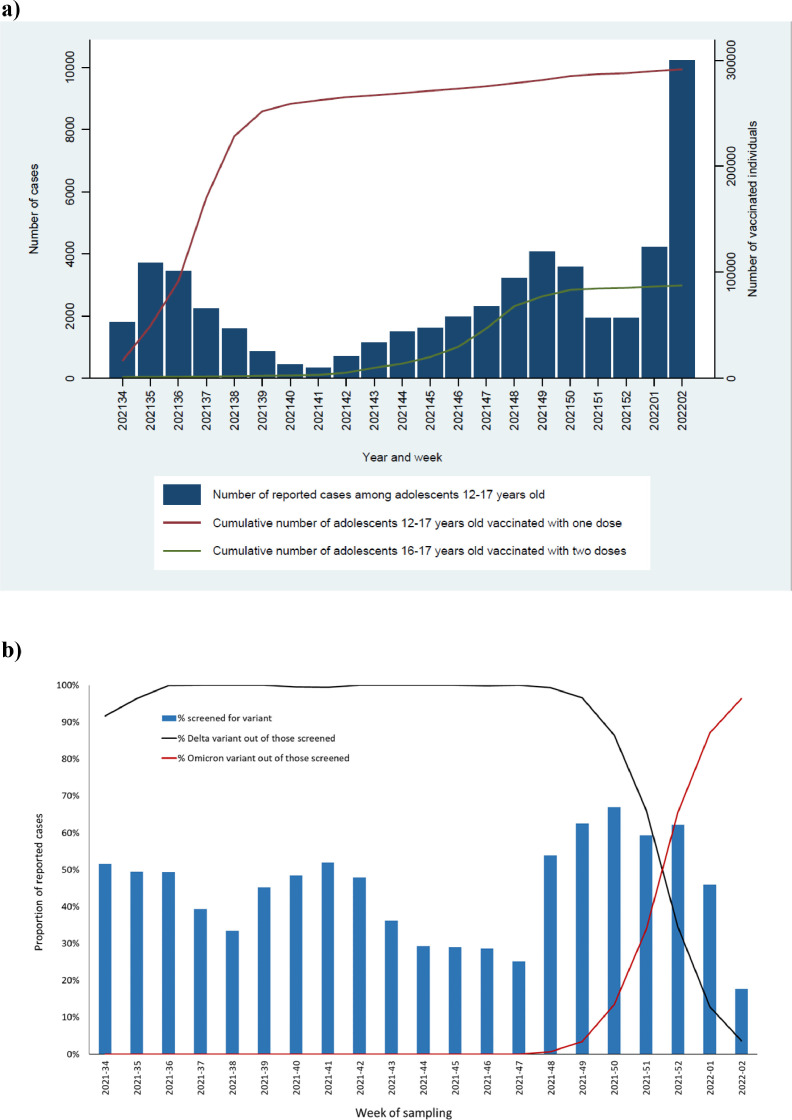
Overall, we included 372,179 adolescents (aged 12-17 years), of whom 52,891 reported SARS-CoV-2 infections by the end of the study period. Of the 52,891 reported cases, 42% were screened for variants and from those 26% of variants were sequenced. The first Omicron case in this population was detected on December 2, 2021 (week 48). The proportion of cases screened for variants varied throughout the study period, with the highest proportion (67%) in week 50 after the first Omicron cases in Norway (Figure 1 ). Overall, 17,103 infections were identified as Delta and 5,121 as Omicron. Further population characteristics are presented in Table 1 .
Figure 1.
Number of COVID-19 reported cases by week of sampling and cumulative number of vaccinated adolescents 12-17 years old by vaccine doses received and week (a); proportion of reported COVID-19 cases among adolescents 12-17 years old with data on virus variant, and proportion of Delta and Omicron by week of sampling (b), Norway, August 25, 2021- January 16, 2022 (n = 52,891).
Table 1.
Characteristics of the study population and of those who tested positive for SARS-CoV-2 Delta and Omicron variant, Norway, 25 August 2021- 16 January 2022 (n = 372,179).
| Reported infections |
||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Study populationa (n = 372,179) |
All infections (n = 52,891) |
Delta variant (n = 17,103) |
Omicron variant (n = 5,121) |
||||
| n | % | n | % | n | % | n | % | |
| Sex | ||||||||
| Male | 191,316 | 51.4 | 26,967 | 51.0 | 8,681 | 50.8 | 2,573 | 50.2 |
| Female | 180,863 | 48.6 | 25,924 | 49.0 | 8,422 | 49.2 | 2,548 | 49.8 |
| Age groups in years | ||||||||
| 12-15 | 254,498 | 68.4 | 37,993 | 71.8 | 11,738 | 68.6 | 4,065 | 79.4 |
| 16-17 | 117,681 | 31.6 | 14,898 | 28.2 | 5,365 | 31.4 | 1,056 | 20.6 |
| Country of birth | ||||||||
| Norway | 329,590 | 88.6 | 45,149 | 85.4 | 14,623 | 85.5 | 4,337 | 84.7 |
| Outside of Norway | 42,583 | 11.4 | 7,742 | 14.6 | 2,480 | 14.5 | 784 | 15.3 |
| Unknown | 6 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Risk of severe COVID-19b | ||||||||
| Low | 342,775 | 92.1 | 48,987 | 92.6 | 15,853 | 92.7 | 4,737 | 92.5 |
| Medium | 27,308 | 7.3 | 3,691 | 7.0 | 1,177 | 6.9 | 362 | 7.1 |
| High | 2,096 | 0.6 | 213 | 0.4 | 73 | 0.4 | 22 | 0.4 |
Note that the study population in the main analysis includes the individuals at the beginning of the study period (August 25, 2021) and that the population in the sub analyses for the period November 2021 to January 2022 is different since prior infections are excluded and few people died.
Risk of severe disease based on underlying comorbidities that are associated with a moderate or high risk of serious illness regardless of age. Details on the definitions used are provided in the Supplement, part 1.
At the start of the study period (end of week 33), 3,445 adolescents had received one vaccine dose, and of those aged 16-17 years, 1,121 received a second dose. Vaccination uptake increased during the study period, and when Omicron started circulating (week 48), the vaccine coverage was approximately 75% (278,422 /372,179) for one dose among those aged 12-15 years and 59% (67,955 /117,681) for two doses among those aged 16-17 years. By the end of the study period, the vaccine coverage was 78% (291,511/372,179), with one dose among those aged 12-15 years and 74% (87,613/117,681) with two doses among those aged 16-17 years (Figure 1).
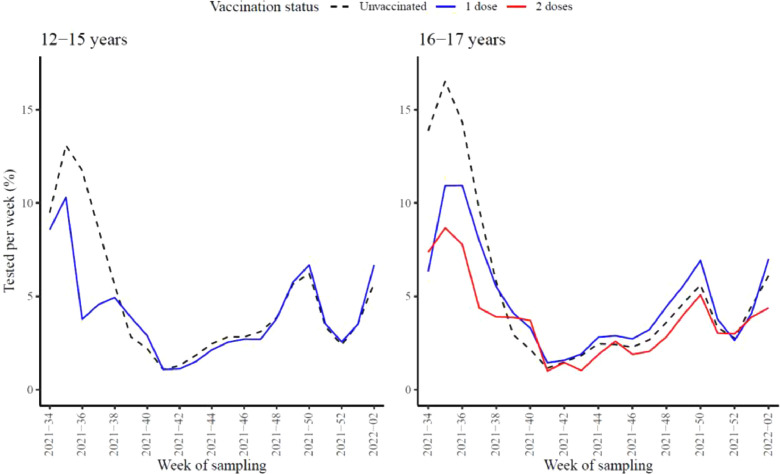
Testing activity was similar among unvaccinated and vaccinated adolescents and among the respective groups for the two age groups throughout the study period. The proportion of individuals tested per week ranged from 1.1% to 16.5% for the unvaccinated adolescents, 1.1% to 10.9% for those vaccinated with one dose, and 1.0% to 8.7% for those vaccinated with two doses (Figure 2 ).
Figure 2.
Proportion of individuals tested per week for the age group 12-15 (left panel) and 16-17 (right panel) years by vaccine status (unvaccinated: black dashed line, one dose: blue line, two doses: red line), Norway, August 25, 2021-January 16, 2022 (n = 372,179).
Vaccine Effectiveness
Delta variant
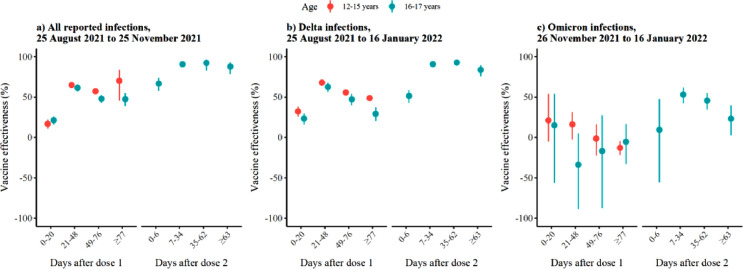
The first period (21-48 days) after one dose the VE against Delta infections was 68% (95% confidence interval [CI], 64-71%) and 63% (95% CI: 56-68%) among those aged 12-15 years and 16-17 years, respectively. The VE decreased to 49% (95% CI: 46-52%) and 29% (95% CI: 20-37%) after 77 days or more, respectively (Figure 3 , Supplementary Table S2). Among those aged 16-17 years, that received two doses, the VE was 91% (95% CI: 89.1-92%) 7-34 days after the second dose compared with 93% (95% CI: 90-95%) 35-62 days and 84% (95% CI: 76-89.0%) after 63 days or more (Figure 3, Supplementary Table S2). These analyses showed a decrease in VE with time since the last vaccination (no overlapping CI). We also estimated the overall VE for the period after 21 days from dose one, which was 54% (95% CI: 52-56%) for those aged 12-15 years and 45% (95% CI: 40-50%) for those aged 16-17 years. The overall VE after 7 days from dose two was 91% (95% CI: 90-92) for those aged 16-17 years.
Figure 3.
Adjusted vaccine effectiveness for age 12-15 years (orange) and 16-17 (blue) by vaccination status against (a) all reported infections from August 25 to November 25, (b) reported Delta infections from August 25, 2021 to January 16, 2022, and (c) reported Omicron infections from November 26 to January 16. We estimated adjusted vaccine effectiveness using Cox regression stratified by age, sex, county of residence, country of birth, crowding and underlying comorbidities. Details on the numbers presented here are included in supplementary Table S2.
Note 1: The vaccine effectiveness in 0-6 days after the second dose probably reflects the effect of the first vaccine dose.
Note 2: Overall, during November 26, 2021 to January 16, 2022: There were 8,013 Delta infections reported, of which 8,011 were included in the Cox regression (two cases had date of event before date of entry due to errors). From those 8,011 Delta cases, the 6,507 were in age group 12-15 and 1,504 in the age group 16-17 years. Moreover, there were 5,123 Omicron infections of which 5,120 were included in the Cox regression (three cases had date of event before date of entry due to errors). From those 5,120 Omicron cases, the 4,064 were in age group 12-15 and 1,056 in the age group 16-17.
The VE estimates were similar when we conducted these analyses using all reported infections during the period August 25, 2021 to November 25, 2021 (Delta dominating), with slightly higher estimates for 63 days or more after the second dose (88% vs 84% with overlapping CI). The decrease of VE was less clear including this period, likely due to shorter follow-up. The estimates for VE were significantly higher 77 days or more after one dose when considering all infections compared with Delta (48% vs 29% with no overlapping CI). This could be possibly explained by a longer follow-up period in the analysis using Delta cases (up to January 16, 2022) than the analysis for all infections (up to November 25, 2021).
Omicron variant
Among those aged 16-17 years who received two doses, the VE against Omicron infection peaked 7-34 days after the second dose at 53% (95% CI: 43-62%) and substantially decreased to 23% (95% CI: 3-40%) 63 days or more after the second dose (no overlapping CI) (Figure 3, Supplementary Table S2). For both age groups, we did not observe a protective effect against Omicron SARS-CoV-2 infection after one dose. The VE against Omicron infection was significantly lower than Delta in all respective time points (no overlapping CI) after receiving the second dose of vaccine in those aged 16-17 years. We also did not observe a protective effect against Omicron when we estimated the overall VE for the period after 21 days from dose one, which was -11% (95% CI: -19‒ -3%) for those aged 12-15 years and -12% (95% CI: -38‒8%) for those aged 16-17 years. The overall VE after 7 days from dose two was 46% (95% CI: 36-54) for those aged 16-17 years.
In our study, we estimated the VE against infection, and we did not include any VE analyses against hospitalization or death (severe disease) due to the small numbers of events. During the study period, there were 31 hospitalizations, with COVID-19 as the main reason for admission, and four intensive care unit admissions among adolescents aged 12 to 17 years, from which none died.
Discussion
We found that two doses of BNT162b2 vaccine were highly effective against any (irrespective of symptoms) SARS-CoV-2 Delta infection and less effective against Omicron infection among adolescents aged 16-17 years. We did not observe a protective effect against Omicron SARS-CoV-2 infection after one dose but observed some protection against Delta infection. Overall, the VE against Omicron was lower than against Delta. For both variants and in both age groups, the VE against infection decreased with time since vaccination.
The high VE (>90%) against the Delta variant shortly after the second dose is in line with results from other studies among adolescents in Israel, United States, Singapore, and England [8,10,[13], [14], [15]. Our results are aligned with several studies reporting reduced VE against infections with Omicron compared to Delta variants in adolescents and adults [13,14,[21], [22], [23]. We did not observe a protective effect against Omicron infection after receiving one dose of the vaccine, but a study among adolescents in England reported a 53% effectiveness against symptomatic Omicron infection 21-27 days after vaccination [14], and another study in Singapore reported an overall 22% effectiveness against confirmed Omicron infection [13]. Our estimated VE against Omicron infection after two doses was also lower than the estimates from England; 53% compared with over 70% in 7-34 days after the second dose respectively [14]. However, the trends were comparable with lower effectiveness against Omicron than Delta variant and decreasing effectiveness with time since the last vaccine dose [14]. A possible explanation for the differences is the high rates of asymptomatic infection for Omicron than other variants of concern [24] and increased VE against symptomatic infections, as well as differences in health-seeking behavior. In our study, we included a period with similar testing strategy, which was independent of vaccine status, to limit this bias. We did not observe differences in the proportions of tested by week over the study period by vaccine status (Figure 2). Because the population, study design, interval between doses, the period, outcomes, and epidemic are site- and study-dependent, it is important to have data from various settings to compare and guide vaccine policies.
Similar to data reported in adults, the vaccine-induced protection against infection wanes with time since last dose for both variants [21]. Waning against Delta infection among adolescents has been reported previously [8,13,22,25]. In addition, the studies from England and Qatar suggested that the protection against Omicron infection wanes faster than Delta infections and to infections in the pre-Omicron period, respectively [14,26]. The pooled results from a metanalysis that included 14 studies in children/adolescents that demonstrated that the second dose, as well as the booster vaccine, were more effective than the first dose in protecting children and adolescents from the Omicron infection, especially those with symptomatic COVID-19 [16].
In our study, we did not include any VE analyses against severe disease, such as hospitalization or death, due to small number of events. However, studies in both adults and adolescents have shown stronger effectiveness and longer duration against more severe disease than infection [13,21,27]. Because adolescents have a low risk of severe disease, the necessity to prevent infection in previously primary vaccinated adolescents is still uncertain.
The access and ability to link information on an individual level using data collected in various national systems is a great advantage and allows us to estimate the national VE and in subpopulations, such as adolescents. However, using register-based data comes with some limitations that should be considered when interpreting the results. Data used from the registries are not collected for the purpose of this study, and therefore, it is not guided by the current study, as would be the case for independent data gathering, which can affect the focus on level of detail, error checking, and precision in the available data. However, there is no reason to believe that such discrepancies, if they exist, would differ among the different groups that we have compared, and our estimates in the study and previously published work are in line with that reported by others. Various aspects related to disease dynamics, risk of infection, testing strategy, and response changed over the study period, making it challenging to identify all elements that affect VE and risk of infection. However, we expect these changes to be similar between the adolescents in this study and by using time explicitly in our models, we take some of these changes indirectly into consideration. The sensitivity analyses using data from only cases screened for variants compared with all reported cases provided similar estimates on VE (Supplementary Table S3). We conclude that using all reported SARS-CoV-2 cases in periods with one variant dominating can provide similar VE estimates with a larger power when only a small proportion of samples are screened.
In Norway, SARS-CoV-2 testing during the study period was free and readily available for everyone. In addition, in areas and periods with high infection rates, regular testing was recommended for adolescents attending school. However, it is likely that not all individuals infected with SARS-CoV-2 have been detected, which can affect the estimates if the fraction of unidentified cases differs by vaccine status. Moreover, the estimated VE can be affected if the number and types of social contacts by an individual depends on his/her vaccination status [28], for example, if getting vaccinated results in behavioral changes associated with higher risk of exposure (underestimate) and/or lower likelihood of testing (overestimate). However, we expect in this study population that risk behavior, type of contacts, and testing would be relatively homogenous because this population had similar exposures (i.e., being at school), testing requirements did not depend on vaccination status, and we did not observe any big differences in the proportion of individuals tested per week in the different vaccination status groups.
In conclusion, one and two doses of BNT162b2 among adolescents protected well against Delta SARS-CoV-2 infection, with higher protection among those with two doses. We did not observe a protective effect against Omicron SARS-CoV-2 infection after one vaccine dose, but we observed a moderate protection after two doses. VE decreased with time since vaccination for both variants, and we observed a steeper decrease in the effectiveness against Omicron infection than Delta from the time since vaccination with two doses. These results suggest that vaccination might have limited effect in preventing Omicron infection among adolescents that can also result in challenges limiting the spread. Evidence from other studies suggest that even if vaccination provides modest protection against Omicron infection (symptomatic or not), the protection against severe disease seems to remain. Given the low risk of severe disease for children and adolescents with the currently circulating strains of SARS-CoV-2 and the vaccines’ limited effect against infection and transmission, the impact of further vaccination of healthy adolescents presently seems low. However, adolescents with severe underlying conditions may have a higher risk of hospitalization for COVID-19. In Norway, children and adolescents with severe comorbidities (e.g., severely immunocompromised; severe neurological, cardiac, or respiratory conditions) are recommended to receive primary and booster vaccination to reduce the risk of severe disease and hospitalization. COVID-19 VE should be monitored and re-evaluated, especially with the introduction of new vaccines, as well as emergence of new variants, because protection might differ from the effectiveness against Delta and Omicron infections in earlier periods.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study was performed as part of routine work at the Norwegian Institute of Public Health.
Ethical approval
Ethical approval was granted by the Regional Committees for Medical and Health Research Ethics (REC) Southeast (reference number 122745).
Acknowledgments
The authors wish to thank all those who have helped to collect and report data to the national emergency preparedness registry at the Norwegian Institute of Public Health (NIPH) throughout the pandemic. The authors are grateful to all health professionals who contributed to vaccinating the Norwegian population and those performing millions of laboratory tests for COVID-19. The authors also thanks the staff at the regional laboratories and the Virology and Bacteriology departments at NIPH involved in the analyses of samples, national variant identification, and whole genome analysis of SARS-CoV-2 viruses. The authors would also like to acknowledge their colleagues at the NIPH who have contributed to the data cleaning from different registries throughout the pandemic.
Author contributions
LV, JDB, SVW, MGI, and HM were involved in the conceptualization of the study. LV and HM drafted the study protocol with feedback from JDB, SVW, JS, MGI. LV, and HM coordinated the study. LV, JS, PL, and HM had directly assessed and verified the underlying data and produced figures and tables. HB cleaned and supported the acquisition of the variant data. LV and HM had full access to all the data and take the responsibility for the integrity of the data and the accuracy of the data analysis. LV conducted the statistical analysis in consultation with HM. All co-authors contributed to the interpretation of the results. LV drafted the manuscript with support from JDB and HM. All co-authors contributed to the revision of the manuscript and approved the final version for submission. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.03.004.
Appendix. Supplementary materials
References
- 1.Bailey LC, Razzaghi H, Burrows EK, Bunnell HT, Camacho PEF, Christakis DA, et al. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175:176–184. doi: 10.1001/jamapediatrics.2020.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kildegaard H, Lund LC, Højlund M, Stensballe LG, Pottegård A. Risk of adverse events after Covid-19 in Danish children and adolescents and effectiveness of BNT162b2 in adolescents: cohort study. BMJ. 2022;377 doi: 10.1136/bmj-2021-068898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontanet A, Grant R, Greve-Isdahl M, Sridhar D. Covid-19: keeping schools as safe as possible. BMJ. 2021;372 doi: 10.1136/bmj.n524. n524. [DOI] [PubMed] [Google Scholar]
- 5.Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021;385:2241–2251. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frenck RW, Jr Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Stavi CJ, Magen O, Barda N, Yaron S, Peretz A, Netzer D, et al. BNT162b2 vaccine effectiveness against omicron in children 5 to 11 years of age. N Engl J Med. 2022;387:227–236. doi: 10.1056/NEJMoa2205011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glatman-Freedman A, Hershkovitz Y, Kaufman Z, Dichtiar R, Keinan-Boker L, Bromberg M. Effectiveness of BNT162b2 vaccine in adolescents during outbreak of SARS-CoV-2 Delta variant infection, Israel, 2021. Emerg Infect Dis. 2021;27:2919–2922. doi: 10.3201/eid2711.211886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutrick K, Rivers P, Yoo YM, Grant L, Hollister J, Jovel K, et al. Interim estimate of vaccine effectiveness of BNT162b2 (Pfizer-BioNTech) vaccine in preventing SARS-CoV-2 infection among adolescents aged 12–17 years - Arizona, July–December 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1761–1765. doi: 10.15585/mmwr.mm705152a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson SM, Newhams MM, Halasa NB, Price AM, Boom JA, Sahni LC, et al. Effectiveness of Pfizer-BioNTech mRNA vaccination against COVID-19 hospitalization among persons aged 12–18 years - United States, June–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1483–1488. doi: 10.15585/mmwr.mm7042e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan SHX, Cook AR, Heng D, Ong B, Lye DC, Tan KB. Effectiveness of BNT162b2 vaccine against omicron in children 5 to 11 years of age. N Engl J Med. 2022;387:525–532. doi: 10.1056/NEJMoa2203209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiew CJ, Premikha M, Chong CY, Wei WE, Ong B, Lye DC, et al. Effectiveness of primary series and booster vaccination against SARS-CoV-2 infection and hospitalisation among adolescents aged 12–17 years in Singapore: a national cohort study. Lancet Infect Dis. 2023;23:177–182. doi: 10.1016/S1473-3099(22)00573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell AA, Kirsebom F, Stowe J, McOwat K, Saliba V, Ramsay ME, et al. Effectiveness of BNT162b2 against COVID-19 in adolescents. Lancet Infect Dis. 2022;22:581–583. doi: 10.1016/S1473-3099(22)00177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price AM, Olson SM, Newhams MM, Halasa NB, Boom JA, Sahni LC, et al. BNT162b2 protection against the omicron variant in children and adolescents. N Engl J Med. 2022;386:1899–1909. doi: 10.1056/NEJMoa2202826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Liang H, Ding X, Cao Y, Yang D, Duan Y. Effectiveness of COVID-19 vaccine in children and adolescents with the Omicron variant: a systematic review and meta-analysis. J Infect. 2023;86 doi: 10.1016/j.jinf.2023.01.001. e64–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norwegian Institute of Public Health. Coronavirus vaccine - information for the public, https://www.fhi.no/en/id/vaccines/coronavirus-immunisation-programme/coronavirus-vaccine/; 2021 [accessed 05 November 2022].
- 18.Brandal LT, MacDonald E, Veneti L, Ravlo T, Lange H, Naseer U, et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.50.2101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norwegian Institute of Public Health. Emergency preparedness register for COVID-19 (Beredt C19), https://www.fhi.no/en/id/infectious-diseases/coronavirus/emergency-preparedness-register-for-covid-19/; 2021 [accessed 05 November 2022].
- 20.Norwegian Institute of Public Health. Påvisning og overvåkning av SARS-CoV-2-virusvarianter, https://www.fhi.no/nettpub/coronavirus/testing/pavisning-og-overvakning-av-sars-cov-2-virusvarianter/; 2021 [accessed 05 November 2022].
- 21.Buchan SA, Chung H, Brown KA, Austin PC, Fell DB, Gubbay JB, et al. Estimated effectiveness of COVID-19 vaccines against omicron or Delta symptomatic infection and severe outcomes. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen CH, Schelde AB, Moustsen-Helm IR, Emborg H-D, Krause TG, Mølbak K, et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. medRxiv. 2021 https://www.medrxiv.org/content/10.1101/2021.12.20.21267966v1 22 December. [accessed 20 March 2023] [Google Scholar]
- 23.Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022;28:1063–1071. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrett N, Tapley A, Andriesen J, Seocharan I, Fisher LH, Bunts L, et al. High rate of asymptomatic carriage associated with variant strain omicron. medRxiv. 14 January 2022 https://www.medrxiv.org/content/10.1101/2021.12.20.21268130v2 [accessed 20 March 2023] [Google Scholar]
- 25.Prunas O, Weinberger DM, Pitzer VE, Gazit S, Patalon T. Waning effectiveness of the BNT162b2 vaccine against infection in adolescents. Clin Infect Dis. 2023;76:113–118. doi: 10.1093/cid/ciac315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chemaitelly H, AlMukdad S, Ayoub HH, Altarawneh HN, Coyle P, Tang P, et al. Covid-19 vaccine protection among children and adolescents in Qatar. N Engl J Med. 2022;387:1865–1876. doi: 10.1056/NEJMoa2210058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordström P, Ballin M, Nordström A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. Lancet. 2022;399:814–823. doi: 10.1016/S0140-6736(22)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haber M, Halloran ME, Longini IM, Watelet L. Estimation of vaccine efficacy in non-randomly mixing populations. Biom J. 1995;37:25–38. doi: 10.1002/bimj.4710370103. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.