Abstract
Objective:
To investigate the impact of household food insecurity during the third trimester of pregnancy on the growth indicators of infants aged less than 6 months.
Design:
Retrospective longitudinal study.
Setting:
137 healthcare centres (15 cities) in Khorasan Razavi province, Iran. Data were extracted from the Sina Electronic Health Record System (SinaEHR®).
Participants:
This study was conducted on 2481 mother and infant dyads during November 2016–March 2019. The Household Food Insecurity Access Scale (nine-item version) was used to measure food insecurity in the third trimester of pregnancy. Women who delivered singleton infants were included in the study, and anthropometric indices of infants were measured throughout the first 6 months of life.
Results:
Approximately 67 % of the participants were food secure, while 33 % had varying degrees of food insecurity. The children born to the mothers in the food-insecure households were, respectively, 2·01, 3·03, and 3·83 times more likely to be stunted at birth (95 % CI 1·17, 3·46), 4 months (95 % CI 1·21, 7·61) and 6 months of age (95 % CI 1·37, 10·68) compared to their counterparts in the food-secure households. However, there were no significant differences in mean birth weight, birth height and head circumference at birth between the two groups.
Conclusions:
Household food insecurity during pregnancy is a risk factor for stunting in infants aged less than 6 months. Therefore, national nutrition programs could considerably support women in food-insecure households during and before pregnancy.
Keywords: Food Insecurity, Pregnancy, Infants, Stunting
Food security is defined as the physical, social and economic accessibility of sufficient, safe and nutritious foods all the times for people to meet their dietary needs and food preferences for active and healthy life(1). In 2018, the FAO report showed that approximately two billion people across the world are disposed to moderate or severe food insecurity(2). In Iran, the prevalence rate of food insecurity among households, mothers and children has been reported to be 49, 61 and 67 %, respectively, with an increasing trend during 2004–2015(3).
It is believed that food insecurity mainly affects underprivileged societies, young children and women of the reproductive age(4,5). Ample evidence suggests that food insecurity is associated with poor health outcomes in women, including higher risk of obesity(6), higher gestational weight gain(6,7), anaemia(8), depression(9), gestational complications (diabetes, hypertension and birth defects)(8,10,11) and the ultimate increase in the burden of healthcare costs on the community(2). Due to increased nutritional requirements during pregnancy, not reaching the adequate nutrient supply may increase the risk of malnutrition and consequent intrauterine growth restrictions(12,13) which is a significant risk factor for under-five morbidities and mortalities(14).
Extensive research has been focussed on food insecurity, and some of the findings have confirmed the impact of maternal food insecurity on infancy and early childhood growth(15,16). In this regard, a study conducted in the USA indicated that maternal food insecurity was associated with a threefold increase in the delivery of low-birth weight infants(17). Also, living in food-insecure households severely affects the health status of children in later life as they are more prone to malnutrition in growth age. Not only may food insecurity lead to increased odds of childhood obesity(18) and poor cognitive performance(19,20), but it also is associated with psychological distress during their transition into adulthood(21).
Food insecurity is assumed to affect the growth of infants by feeding practices such as breastfeeding. As the most cost-effective approach to meeting the nutritional needs of infants and reducing childhood diseases(22), exclusive breastfeeding in the first 6 months of life is adversely affected by food insecurity through changes in the initiation and duration of breastfeeding(23); therefore, food insecurity seems to adversely impact infant growth variably(9,24). Nevertheless, only limited studies have been focussed on the effect of household food insecurity (HFI) on infant growth indices, proposing conflicting results(25).
This study aimed to investigate the effects of HFI during the third trimester of pregnancy on the growth indicators of infants aged less than 6 months.
Materials and Methods
Study design, sampling and inclusion criteria
In this retrospective longitudinal study, data were collected from Sina Electronic Health System (SinaEHR®) during November 2016–March 2019. SinaEHR is an integrated health information system supervised by Mashhad University of Medical Sciences, which contains the health records of more than five million people in Khorasan Razavi province, Iran.
The current research participants were selected from 15 cities (137 healthcare centres) in Khorasan Razavi province among women 18–45 years of age who referred to the healthcare centres for routine check-ups during their third trimester of pregnancy. To maximise the target population (registered in SinaEHR), we pooled data between November 2016 and March 2019, and random sampling was used for selecting study participants. We included only women who delivered singleton infants, but mothers with a history of chronic heart diseases, anaemia, type II diabetes mellitus and gestational diabetes were excluded. In addition, we excluded infants with a history of hospitalisation during the first 6 months of life.
Sociodemographic and anthropometric measurements
The sociodemographic and anthropometric data, including age, education level, smoking during pregnancy, medical history of the mother and infant, place of residence and maternal pre-pregnancy BMI were collected using a questionnaire which was developed and applied via the SinaEHR. Anthropometric variables of infants (weight, height, and head circumference at birth, second, fourth and sixth months of life) and their mothers (weight and height) were measured by trained personnel using standard protocol.
Infants’ weight was measured with minimal clothing using a Seca scale with an accuracy of 10 g (Seca 725, Hamburg, Germany). Their height (supine position) and head circumference were also measured using a standard measuring tape with 1 mm’s accuracy. Maternal weight and height were measured with light clothing and no shoes using a Seca scale and a stadiometer (Seca 755, Hamburg, Germany) with an accuracy of 1 mm for height and 100 g for weight.
The infants who weighed less than 2500 g were considered low birthweight, and those weighing ≥4000 g were classified as large for gestational age(26). The WHO Z score system was used to classify the nutritional status, and scores less than −2 of the WHO standards for height-for-age Z scores (HAZ), weight-for-height Z scores and weight-for-age Z scores (WAZ) were considered as stunted, wasted and underweight, respectively. Regarding the weight-for-height Z score standard, infants with the Z scores between −2 and 2 were considered normal, and those with the Z score of above 2 were considered overweight. In addition, infants with the HAZ between the Z-score lines −1 and 2, WAZ between the Z-score lines of −1 and 3 were classified as normal(27).
Food insecurity measurement
HFI was measured using a standard questionnaire (Household Food Insecurity Access Scale, Household Food Insecurity Access Scale), which has been developed by Food and Nutrition Technical Assistance II project in collaboration with others(28,29). The validity of the Persian version of this questionnaire has been confirmed by Salarkia et al., who reported the acceptable internal consistency of the scale for Iranian households with the Cronbach’s alpha of 0·95(30).
The Household Food Insecurity Access Scale is composed of a set of nine items specific to an experience of food insecurity occurring within the previous 4 weeks. Endorsed a standard scoring procedure was used with 1 point for occurrence and 0 for non-occurrence. The frequency scores are within the range of 0–3, with zero indicating non-occurrence, one showing rare occurrence (once/twice in the past 4 weeks), two indicating sometimes (3–10 times in the past 4 weeks) and three for often (>10 times in the past month)(31,32). For the purpose of this article, based on the Household Food Insecurity Access Scale total score (nine items based on the frequency score), the study population was classified as food secure (scores 0–1), mildly food insecure (scores 2–7), moderately food insecure (scores 8–14) and severely food insecure (scores 15–27).
Statistical analysis
Data analysis was performed in SPSS version 22. The continuous variables were expressed as mean and sd, and the categorical variables were expressed as frequency and percentage. Independent samples t-test was used to assess the significant difference in the Z scores between the food-secure and food-insecure subjects, and one-way repeated-measures ANOVA was applied to evaluate the significant difference in the Z scores between various measurement times. The variables were categorised, and the relative risk was calculated at 95 % CI(12). In addition, multinomial logistic regression was applied considering food insecurity as a factor and the covariates of maternal age, pre-pregnancy BMI, education level and parity. In all the statistical analyses, the P-value of less than 0·05 was considered significant.
Results
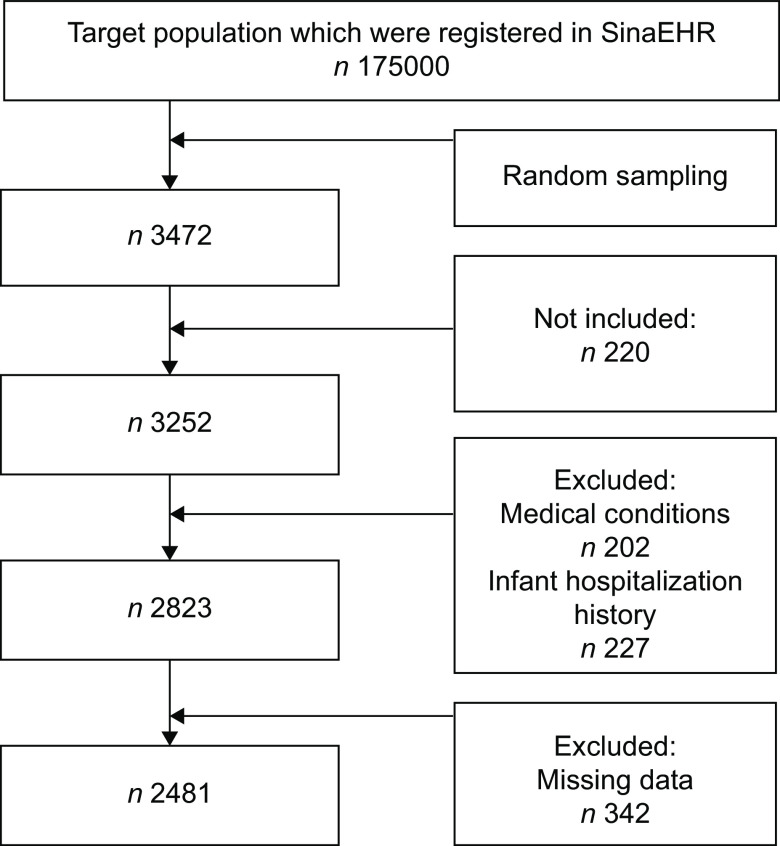
As shown in Fig. 1, more than 175 000 pregnant women were registered in SinaEHR during the study period, and 3474 women were randomly selected for this study. Among these women, 3252 were aged 18–45 years and had live, singleton deliveries. Notably, 202 women were excluded due to medical conditions (e.g. type II diabetes mellitus, gestational diabetes mellitus, anaemia, CHD), 342 women were excluded due to data loss and 227 cases were excluded due to neonatal hospitalisation history.
Fig. 1.
Flowchart of sampling procedure
The sociodemographic characteristics and anthropometric indices of the participants are presented in Table 1. The proportion of the infants by gender was reported at 50 %. In addition, 0·8 % of the women (n 20) had smoking habits within the past 12 months upon enrolment. The majority of study population were urban (95·6 %) and outskirts residents (49·7 %). In terms of education level, 37·2 % of the participants had an academic degree, 35·4 % had a high school diploma and the others had secondary education or were illiterate. Approximately 4 % of the neonates (n 100) were low birth weight, and 4·5 % (n 110) had macrosomia (weight: >4000 g). The infants’ mean birth weight was 3237 g, the mean pre-pregnancy BMI was 26·29 kg/m2 and the mean gravidity was 2·44.
Table 1.
Sociodemographic characteristics of sample population (number of percentage and mean and sd values)
| N (%) | Mean±sd | ||||
|---|---|---|---|---|---|
| Variables | N | % | Mean | sd | |
| Gender | Male | 1280 | 51·6 | ||
| Female | 1201 | 48·4 | |||
| Birth weight (g) | LBW | 100 | 4 | 3237 | 439 |
| Normal | 2270 | 91·5 | |||
| Macrosomia | 111 | 4·5 | |||
| Birth height (cm) | 49·93 | 2·33 | |||
| Birth head circumference (cm) | 34·74 | 1·74 | |||
| Maternal age (year) | 29·52 | 5·78 | |||
| Pre-pregnancy BMI | 26·29 | 5·40 | |||
| Mode of delivery | C-section | 1163 | 46·9 | ||
| Natural | 1318 | 53·1 | |||
| Premature birth | 42 | 1·7 | |||
| Gravidity | 2·44 | 1·39 | |||
| Exclusive breastfeeding | 1988 | 80·1 | |||
| Smoker mothers | 20 | 0·8 | |||
| Maternal education level | Academic | 923 | 37·2 | ||
| Diploma | 878 | 35·4 | |||
| Below Diploma | 680 | 27·4 | |||
| Place of residence | Rural | 110 | 4·4 | ||
| Town (<20 K) | 13 | 0·5 | |||
| Town (20–50 K) | 164 | 6·6 | |||
| Town (50–500 K) | 82 | 3·3 | |||
| Outskirts | 1233 | 49·7 | |||
| City centre | 879 | 35·4 | |||
As presented in Table 2, the highest prevalence of wasting was observed at birth, which gradually decreased with infants’ growth. The comparison of the mean Z scores between the food-secure and food-insecure subjects using independent t-test (Table 3) indicated that birthweight, birth height, head circumference at birth and birth’s Z scores were not significantly different between groups, although the infants in food-secure households had lower WAZ at 6 months of age (P = 0·003) and lower HAZ at the age of 2, 4 and 6 months (P ≤ 0·001).
Table 2.
Prevalence of classified levels of Z scores (number of percentage (%) values)
| Birth N (%) | Two Months N (%) | Four Months N (%) | Six Months N (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | N | % | N | % | N | % | N | % | |
| WHZ | Wasted | 203 | 8·2 | 102 | 4·1 | 74 | 3 | 62 | 2·5 |
| Normal | 2238 | 90·1 | 2295 | 92·4 | 2320 | 93·5 | 2320 | 93·5 | |
| Overweight | 40 | 1·6 | 84 | 3·4 | 87 | 3·5 | 99 | 4 | |
| WAZ | Underweight | 67 | 2·7 | 72 | 2·9 | 44 | 1·8 | 40 | 1·6 |
| Normal | 2374 | 95·7 | 2335 | 94·1 | 2355 | 94·9 | 2342 | 94·4 | |
| Other | 40 | 1·6 | 74 | 3 | 82 | 3·3 | 99 | 4 | |
| HAZ | Stunted | 97 | 3·9 | 77 | 3·1 | 30 | 1·2 | 27 | 1·1 |
| Normal | 2377 | 95·8 | 2384 | 96·1 | 2436 | 98·1 | 2432 | 98 | |
| Other | 7 | 0·3 | 20 | 0·8 | 15 | 0·6 | 22 | 0·9 | |
Z scores of HAZ, WHZ and WAZ blow −2 considered stunted, wasted and underweight, respectively; WHZ of −2 to 2 considered normal and >2 classified as overweight; HAZ of −1 to 2 and WAZ of −1 to 3 considered normal. HAZ, height-for-age Z scores; WAZ, weight-for-age Z scores; WHZ, weight-for-height Z scores.
Table 3.
Independent samples t-test for comparison of Z scores in food-secure and food-insecure subjects (mean and sd values)
| Characteristics | Food-secure | Food-insecure | t | df | P-value | ||
|---|---|---|---|---|---|---|---|
| Mean ± sd | Mean ± sd | ||||||
| Mean | sd | Mean | sd | ||||
| Birth weight (cm) | 3247 | 431 | 3218 | 456 | −1·5 | 2479 | 0·054 |
| Birth height (cm) | 49·96 | 2·25 | 49·88 | 2·49 | −0·76 | 2453 | 0·057 |
| Birth head circumference (cm) | 34·75 | 1·68 | 34·72 | 1·86 | −0·52 | 2436 | 0·177 |
| Birth WHZ | −0·40 | 1·17 | −0·40 | 1·20 | 0·04 | 1893 | 0·971 |
| 2-month WHZ | −0·05 | 1·12 | 0·00 | 1·14 | 1·14 | 2375 | 0·254 |
| 4-month WHZ | −0·05 | 1·12 | 0·02 | 1·05 | 1·32 | 2310 | 0·186 |
| 6-month WHZ | 0·10 | 1·10 | 0·09 | 1·06 | −0·33 | 1854 | 0·745 |
| Birth WAZ | −0·16 | 0·87 | −0·21 | 0·94 | −1·22 | 1234 | 0·221 |
| 2-month WAZ | 0·09 | 0·98 | 0·01 | 1·03 | −1·83 | 2367 | 0·067 |
| 4-month WAZ | 0·18 | 1·02 | 0·10 | 1·01 | −1·79 | 2311 | 0·073 |
| 6-month WAZ | 0·27 | 1·02 | 0·12 | 1·01 | −2·92 | 1848 | 0·003 |
| Birth HAZ | −0·08 | 1·05 | −0·16 | 1·11 | −1·56 | 1891 | 0·120 |
| 2-month HAZ | 0·24 | 1·17 | 0·05 | 1·25 | −3·59 | 2379 | <0·001 |
| 4-month HAZ | 0·40 | 1·08 | 0·23 | 1·12 | −3·46 | 2311 | 0·001 |
| 6-month HAZ | 0·47 | 1·09 | 0·23 | 1·10 | −4·43 | 1853 | <0·001 |
HAZ, height-for-age Z scores; WAZ, weight-for-age Z scores; WHZ, weight-for-height Z scores.
P < 0·05 considered statistically significant.
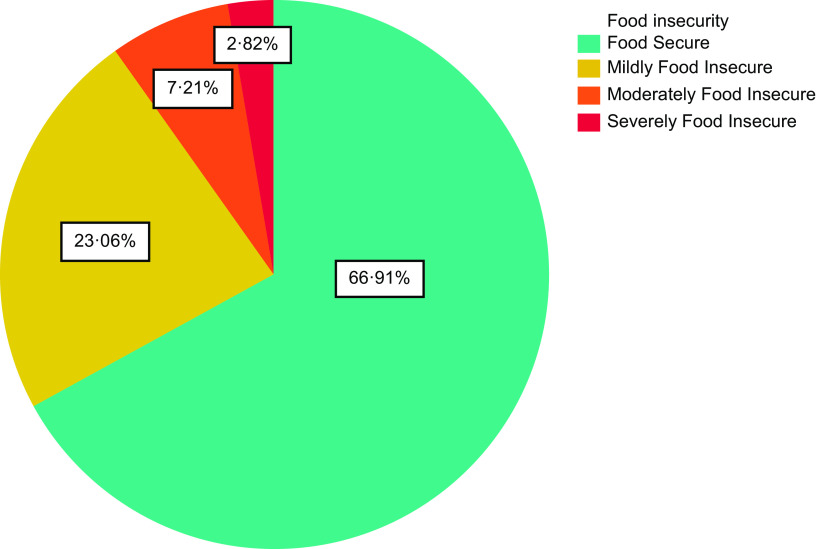
Figure 2 shows the prevalence of food insecurity in the sample population. As illustrated herewith, approximately two-thirds of the participants were food secure, while the prevalence of mild, moderate and severe food insecurity was 23 % (n 572), 7 % (n 179) and 3 % (n 70), respectively. Table 4 shows the relative risk of HFI during pregnancy for the anthropometric characteristics of the infants. Accordingly, the children born to the women in food-insecure households were, respectively, 1·96, 2·72, and 3·87 times more likely to be stunted at birth (95 % CI 1·23, 3·12), 4 months (95 % CI 1·28, 5·77) and 6 months of age (95 % CI 1·54, 9·75) compared to the infants of the food-secure women, while they were also 1·85 times more likely to be underweight at the age of 4 months (95 % CI 1·01, 3·41). After adjustment for pre-pregnancy BMI, age, education level, parity, smoking habits and place of living, the risk of stunting remained high at birth (adjusted relative risk (aRR) = 2·01; 95 % CI 1·17, 3·46), 4 months (aRR = 3·03; 95 % CI 1·21, 7·61), and 6 months of age (aRR = 3·83; 95 % CI 1·37, 10·68) in the infants born in food-insecure households compared to those in food-secure households. On the other hand, food insecurity was not significantly associated with weight-for-height Z score and WAZ during the first 6 months of life, and no correlation was observed with birth weight. These results remained persistent after adjustment for potential confounders.
Fig. 2.
Prevalence of household food insecurity during pregnancy
Table 4.
Unadjusted and adjusted relative risk of household food insecurity during pregnancy in anthropometric characteristics of neonates (number of percentage (%) and RR and 95 % CI values)
| Unadjusted | Adjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Food security (%) | RR (95 % CI | RR (95 % CI) | |||||||
| Characteristics | No (%) | Yes (%) | RR | 95 % CI | P-value | RR | 95 % CI | P-value | |
| Birth weight | LBW | 4·6 | 3·7 | 1·26 | 0·83, 1·90 | 0·274 | 1·10 | 0·67, 1·83 | 0·706 |
| Normal | 90·5 | 92 | Referent | ||||||
| Macrosomia | 4·9 | 4·3 | 1·16 | 0·78, 1·72 | 0·470 | 1·22 | 0·78, 1·90 | 0·378 | |
| Birth WHZ | Wasted | 8·3 | 8·4 | 0·96 | 0·68, 1·35 | 0·180 | 0·94 | 0·64, 1·37 | 0·746 |
| Normal | 90 | 90·2 | Referent | ||||||
| Overweight | 2·4 | 1·4 | 1·38 | 0·67, 2·83 | 0·382 | 1·02 | 0·47, 2·20 | 0·958 | |
| 2-month WHZ | Wasted | 4 | 4·2 | 0·95 | 0·61, 1·47 | 0·821 | 0·95 | 0·59, 1·53 | 0·839 |
| Normal | 92 | 92·6 | Referent | ||||||
| Overweight | 4·0 | 3·2 | 1·25 | 0·79, 1·97 | 0·337 | 1·09 | 0·66, 1·79 | 0·750 | |
| 4-month WHZ | Wasted | 2·1 | 3·5 | 0·59 | 0·33, 1·03 | 0·134 | 0·64 | 0·34, 1·20 | 0·159 |
| Normal | 94·7 | 92·9 | Referent | ||||||
| Overweight | 3·3 | 3·6 | 0·90 | 0·57, 1·46 | 0·667 | 0·82 | 0·50, 1·36 | 0·439 | |
| 6-month WHZ | Wasted | 2·5 | 2·5 | 0·99 | 0·53, 1·84 | 0·667 | 1·25 | 0·64, 2·43 | 0·511 |
| Normal | 94·1 | 93·3 | Referent | ||||||
| Overweight | 3·5 | 4·2 | 0·81 | 0·48, 1·35 | 0·413 | 0·88 | 0·50, 1·53 | 0·640 | |
| Birth WAZ | Underweight | 3·4 | 2·3 | 1·47 | 0·84, 2·58 | 0·180 | 1·71 | 0·84, 3·48 | 0·136 |
| Normal | 94·6 | 96·3 | Referent | ||||||
| Other | 2 | 1·4 | 1·48 | 0·72, 3·07 | 0·291 | 1·07 | 0·49, 2·35 | 0·862 | |
| 2-month WAZ | Underweight | 3·5 | 2·6 | 1·34 | 0·82, 2·19 | 0·248 | 1·34 | 0·80, 2·45 | 0·244 |
| Normal | 92·9 | 94·7 | Referent | ||||||
| Other | 3·6 | 2·7 | 1·35 | 0·83, 2·20 | 0·220 | 1·25 | 0·74, 2·12 | 0·412 | |
| 4-month WAZ | Underweight | 2·6 | 1·4 | 1·85 | 1·01, 3·41 | 0·049 | 1·81 | 0·91, 3·61 | 0·094 |
| Normal | 94·3 | 95·1 | Referent | ||||||
| Other | 3·1 | 3·4 | 0·92 | 0·56, 1·51 | 0·743 | 0·85 | 0·51, 1·42 | 0·539 | |
| 6-month WAZ | Underweight | 2·3 | 1·6 | 1·58 | 0·76, 3·27 | 0·271 | 1·56 | 0·71, 3·44 | 0·272 |
| Normal | 94·4 | 94·4 | Referent | ||||||
| Other | 3·5 | 4·3 | 0·82 | 0·49, 1·37 | 0·443 | 0·89 | 0·51, 1·55 | 0·666 | |
| Birth HAZ | Stunted | 5·7 | 3 | 1·96 | 1·23, 3·12 | 0·005 | 2·01 | 1·17, 3·46 | 0·012 |
| Normal | 94·2 | 96·7 | Referent | ||||||
| Other | 0·2 | 0·3 | 0·49 | 0·06, 4·39 | 0·524 | 0·51 | 0·05, 4·98 | 0·559 | |
| 2-month HAZ | Stunted | 3·4 | 2·9 | 4·46 | 0·98, 21·53 | 0·053 | 1·14 | 0·66, 1·96 | 0·632 |
| Normal | 96·3 | 96·1 | Referent | ||||||
| Other | 0·3 | 1 | 3·95 | 0·91, 17·20 | 0·068 | 0·27 | 0·06, 1·23 | 0·092 | |
| 4-month HAZ | Stunted | 2·1 | 0·8 | 2·72 | 1·28, 5·77 | 0·009 | 3·03 | 1·21, 7·61 | 0·018 |
| Normal | 97·4 | 98·5 | Referent | ||||||
| Other | 0·5 | 0·7 | 0·74 | 0·24, 2·33 | 0·608 | 0·56 | 0·15, 2·10 | 0·386 | |
| 6-month HAZ | Stunted | 2·1 | 0·6 | 3·87 | 1·54, 9·75 | 0·004 | 3·83 | 1·37, 10·68 | 0·010 |
| Normal | 97·2 | 98·5 | Referent | ||||||
| Other | 0·7 | 1 | 0·69 | 0·22, 2·16 | 0·529 | 0·85 | 0·25, 2·86 | 0·795 | |
Multinomial logistic regression adjusted for maternal age, pre-pregnancy BMI, education level and parity; P < 0.05 considered statistically significant. HAZ, height-for-age Z scores; LBW, low birth weight; WAZ, weight-for-age Z scores; WHZ, weight-for-height Z scores.
Discussion
Despite the fact that HFI has been associated with several adverse health outcomes among children, there is scarce literature regarding the correlation of HFI during pregnancy with the nutritional status of children. This was the first study to determine the effects of HFI during pregnancy on infants’ growth indicators in Iran. Our findings indicated that the mean WAZ and HAZ were significantly lower in infants aged 6 months born to food-insecure mothers. HFI during pregnancy may be a risk factor for stunting in infants aged less than 6 months. However, no significant association was observed between maternal food insecurity and birth weight in this study.
Our results showed that the children of food-insecure households had lower WAZ and HAZ compared to the food-secure ones, which is consistent with the studies conducted in other low- and middle-income countries, such as South Ethiopia(33), Nepal(34), Brazil(16) and Kenya(15). Another study conducted on children of different age groups in the same region reported that HFI was significantly correlated with the mean height of the infants aged less than 6 years(35). It seems that food insecurity could affect child development in the early stages of life through at least two mechanisms. First, food-insecure mothers have limited access to nutritionally adequate and safe food, which decreases the resources for the proper growth of their infants during pregnancy and breastfeeding(36,37). Another mechanism is via the maternal emotional status, as food insecurity could be the driving force behind the disruption of maternal psychological and hormonal balance, which in turn has an adverse impact on the quality and duration of breastfeeding(38,39).
The current research’s initial finding was that HFI was only associated with child stunting, and no correlations were observed with wasting and underweight. Stunting or chronic malnutrition is often an indication of long-term nutritional deprivation. However, this is an issue of greater magnitude than underweight or wasting, more accurately reflecting the nutritional deficiencies and illnesses that occur in the most critical periods of growth and development in the early stages of life(28). This impaired linear growth, during the first 1000 d of life particularly, tends to remain up to adulthood, which is associated with greater risk of morbidity and mortality(40), poor cognition and educational performance(41) and nutrition-related chronic diseases in adult life(42).
A growing body of evidence shows that HFI is closely linked to greater risk of stunting in early age in developing countries. A study conducted in South Ethiopia reported that children aged 6–59 months living in food-insecure households were 6·7 times more at risk of stunting than their counterparts in food-secure households(33). Likewise, A cross-sectional study on 2591 children aged 0–60 months confirmed the association of HFI with increased risk of stunting even after adjusting for child, mother and household confounders(43). In a multi-country study conducted on 800 households in eight countries, Psaki et al. found that food access insecurity was associated with a significant shift in the distribution of children’s HAZ toward lower values; the risk of stunting increased by 12 % among children from food-insecure households.
Since the growth of infants aged less than 6 months largely depends on the nutritional status of mothers through breastfeeding, the adverse conditions in this period may even deteriorate with the initiation of complementary feeding(44). Hence, our findings highlight this point that there is a clear need to establish early and timely preventive actions to address HFI during pregnancy and the first 6 months after birth.
In contrast to our finding regarding the lack of an association between food insecurity during pregnancy and birth weight, the study by Saeed et al. on 103 pregnant women aged 19–45 years indicated that food-insecure women were at a fivefold increased risk of delivering low birth weight neonates(45). Moreover, a prospective cohort study of 5044 pregnant women showed that severe HFI is a risk factor for the higher rate of low birth weight(46). The discrepancy could be because the method used in the mentioned study to assess the HFI status differed from our research. In addition, the rate of food insecurity in our population was low at baseline.
There are some limitations that should be acknowledged. Firstly, there was a lack of access to reliable data on the households’ income status and the inevitable use of the habitation area as a welfare indicator. Secondly, our study was only focussed on the first 6 months of life to clarify the linkage between food insecurity during pregnancy and the growth indicators of infants when they primarily depended on the maternal nutritional status. Therefore, further longitudinal studies are recommended on various age groups in order to confirm the associations. Notably, this was the first multi-centric study regarding the effects of HFI on the growth indicators of infants aged less than 6 months in Iran.
Conclusion
The results of this longitudinal study indicated that food insecurity during pregnancy might be a risk factor for stunting in infants aged less than 6 months. Our findings could be incorporated into nutritional interventions during and before pregnancy as HFI may have detrimental effects on children’s growth and development in the early stages of life, which could become life-long.
Acknowledgements
Acknowledgements: Hereby, we extend our gratitude to the Student Research Committee of Mashhad University of Medical Sciences for the financial support of this study (grant no: 980127). Financial support: This research was supported by grants from Student Research Committee of Mashhad University of Medical Sciences (grant no: 980 127). Conflict of interest: None declared. Authorship: K.K., G.R. designed the study, F.K.H. contributed to the implementation of the research, E.M.F. analysed the data, K.K., F.K.H., N.M. and Z.S.H. contributed to the writing of the manuscript and S.R.S. contributed to the final edit of manuscript. Ethics of human subject participation: This study was conducted in accordance with the Declaration of Helsinki and all procedures involving research study participants were approved by the Ethics Committee of Mashhad University of Medical Sciences (code: IR.MUMS.MEDICAL.REC.1398·503). Written informed consent was obtained from all subjects.
References
- 1. Grainger M (2010) World summit on food security (UN FAO, Rome, 16–18 November 2009). Dev Pract 20, 740–742. [Google Scholar]
- 2.World Health Organization (2018) The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for Food Security and Nutrition. Rome: Food & Agriculture Organization. [Google Scholar]
- 3. Behzadifar M, Behzadifar M, Abdi S et al. (2016) Prevalence of food insecurity in Iran: a systematic review and meta-analysis. Arch Iran Med 19, 288–294. [PubMed] [Google Scholar]
- 4. Bhattacharya J, Currie J & Haider SJ (2006) Breakfast of champions? The School Breakfast Program and the nutrition of children and families. J Hum Resour 41, 445–466. [Google Scholar]
- 5. Black RE, Victora CG, Walker SP et al. (2013) Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451. [DOI] [PubMed] [Google Scholar]
- 6. Laraia BA, Siega-Riz AM & Gundersen C (2010) Household food insecurity is associated with self-reported pregravid weight status, gestational weight gain, and pregnancy complications. J Am Diet Assoc 110, 692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kazemi F, Masoumi SZ, Shayan A et al. (2020) Prevalence of food insecurity in pregnant women and its association with gestational weight gain pattern, neonatal birth weight, and pregnancy complications in Hamadan County, Iran, in 2018. Agric Food Secur 9, 12. [Google Scholar]
- 8. Anand SS, Hawkes C, de Souza RJ et al. (2015) Food consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system: a report from the workshop convened by the World Heart Federation. J Am Coll Cardiol 66, 1590–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whitaker RC, Phillips SM & Orzol SM (2006) Food insecurity and the risks of depression and anxiety in mothers and behavior problems in their preschool-aged children. Pediatrics 118, e859–e868. [DOI] [PubMed] [Google Scholar]
- 10. Seligman HK, Bindman AB, Vittinghoff E et al. (2007) Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999–2002. J Gen Intern Med 22, 1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seligman HK, Laraia BA & Kushel MB (2010) Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr 140, 304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marangoni F, Cetin I, Verduci E et al. (2016) Maternal diet and nutrient requirements in pregnancy and breastfeeding. An Italian consensus document. Nutrients 8, 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grieger JA & Clifton VL (2015) A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients 7, 153–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woldeamanuel BT & Tesfaye TT (2019) Risk factors associated with under-five stunting, wasting, and underweight based on ethiopian demographic health survey datasets in Tigray region, Ethiopia. J Nutr Metab 2019, 6967170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mutisya M, Kandala NB, Ngware MW et al. (2015) Household food (in)security and nutritional status of urban poor children aged 6 to 23 months in Kenya. BMC Public Health 15, 1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kac G, Schlüssel MM, Pérez-Escamilla R et al. (2012) Household food insecurity is not associated with BMI for age or weight for height among Brazilian children aged 0–60 months. Plos One 7, e45747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borders AE, Grobman WA, Amsden LB et al. (2007) Chronic stress and low birth weight neonates in a low-income population of women. Obstet Gynecol 109, 331–338. [DOI] [PubMed] [Google Scholar]
- 18. Papas MA, Trabulsi JC, Dahl A et al. (2016) Food insecurity increases the odds of obesity among young Hispanic children. J Immigr Minor Health 18, 1046–1052. [DOI] [PubMed] [Google Scholar]
- 19. Nejati F, Nemati M, Rezaei Ardani AR et al. (2017) Investigating the relation of Household Food Security Status and some Socio-economic factors with children Intelligence Quotient in 2016 – Mashhad-Iran. Med J Mashhad Univ Med Sci 60, 691–700. [Google Scholar]
- 20. Khorramrouz F, Doustmohammadian A, Eslami O et al. (2020) Relationship between household food insecurity and food and nutrition literacy among children of 9–12 years of age: a cross-sectional study in a city of Iran. BMC Res Notes 13, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heflin C, Kukla-Acevedo S & Darolia R (2019) Adolescent food insecurity and risky behaviors and mental health during the transition to adulthood. Child Youth Serv Rev 105, 104416. [Google Scholar]
- 22. Schanler RJ, Krebs NF & Mass SB (2013) Breastfeeding Handbook for Physicians. Elk Grove Village: American Academy of Pediatrics. [Google Scholar]
- 23. Webb-Girard A, Cherobon A, Mbugua S et al. (2012) Food insecurity is associated with attitudes towards exclusive breastfeeding among women in urban Kenya. Matern Child Nutr 8, 199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loopstra R & Tarasuk V (2013) What does increasing severity of food insecurity indicate for food insecure families? Relationships between severity of food insecurity and indicators of material hardship and constrained food purchasing. J Hunger Environ Nutr 8, 337–349. [Google Scholar]
- 25. Ahmadihoseini A, Omidvar N, Nematy M et al. (2019) The relationship between food insecurity and anthropometric measures at birth in low income households. Iran J Pediatr 29, e88410. [Google Scholar]
- 26. World Health Organization (2004) International Statistical Classification of Diseases and Related Health Problems: Tabular List. Geneva: World Health Organization. [Google Scholar]
- 27. Group WMGRS (2006) WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl 450, 76. [DOI] [PubMed] [Google Scholar]
- 28. Coates J, Webb P & Houser R (2003) Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide. Washington: Food and Nutrition Technical Assistance, 3. [Google Scholar]
- 29. Coates J, Swindale A & Bilinsky P (2007) Food and Nutrition Technical Assistance Project (FANTA): Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide (v. 3). Washington, DC: Food and Nutrition Technical Assistance Project. [Google Scholar]
- 30. Salarkia N, Abdollahi M, Amini M et al. (2011) Validation and use of the HFIAS questionnaire for measuring household food insecurity in Varamin-2009. Iran J Endocrinol Metab 13, 374–383. [Google Scholar]
- 31. FANTA U (2014) Development of Evidence-Based Dietary Recommendations for Children, Pregnant Women, and Lactating Women Living in the Western Highlands of Guatemala. Washington, DC: FHI 360/FANTA. [Google Scholar]
- 32. Ballard T, Coates J, Swindale A et al. (2011) Household Hunger Scale: Indicator Definition and Measurement Guide. Washington, DC: Food and Nutrition Technical Assistance II Project, FHI, 360, 23. [Google Scholar]
- 33. Betebo B, Ejajo T, Alemseged F et al. (2017) Household food insecurity and its association with nutritional status of children 6–59 months of age in East Badawacho district, South Ethiopia. J Environ Public Health 2017, 6373595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Osei A, Pandey P, Spiro D et al. (2010) Household food insecurity and nutritional status of children aged 6 to 23 months in Kailali District of Nepal. Food Nutr Bull 31, 483–494. [Google Scholar]
- 35. Shahraki SH, Amirkhizi F, Amirkhizi B et al. (2016) Household food insecurity is associated with nutritional status among Iranian Children. Ecol Food Nutr 55, 473–490. [DOI] [PubMed] [Google Scholar]
- 36. Boyd NR & Windsor RA (1993) A meta-evaluation of nutrition education intervention research among pregnant women. Health Educ Q 20, 327–345. [DOI] [PubMed] [Google Scholar]
- 37. Smith CA (1947) Effects of maternal undernutrition upon the newborn infant in Holland (1944–1945). J Pediatr 30, 229–243. [DOI] [PubMed] [Google Scholar]
- 38. Saha KK, Tofail F, Frongillo EA et al. (2010) Household food security is associated with early childhood language development: results from a longitudinal study in rural Bangladesh. Child Care Health Dev 36 309–316. [DOI] [PubMed] [Google Scholar]
- 39. Venu I, van den Heuvel M, Wong JP et al. (2017) The breastfeeding paradox: relevance for household food insecurity. Paediatr Child Health 22, 180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Black RE, Allen LH, Bhutta ZA et al. (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260. [DOI] [PubMed] [Google Scholar]
- 41. Berkman DS, Lescano AG, Gilman RH et al. (2002) Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet 359, 564–571. [DOI] [PubMed] [Google Scholar]
- 42. Dewey KG & Begum K (2011) Long-term consequences of stunting in early life. Matern Child Nutr 7, Suppl. 3, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sreeramareddy CT, Ramakrishnareddy N & Subramaniam M (2015) Association between household food access insecurity and nutritional status indicators among children aged < 5 years in Nepal: results from a national, cross-sectional household survey. Public Health Nutr 18, 2906–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stewart CP, Iannotti L, Dewey KG et al. (2013) Contextualising complementary feeding in a broader framework for stunting prevention. Matern Child Nutr 2, 27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saeed A, Naqvi M & Javed A (2017) Effects of maternal food insecurity on birth weight of neonates: A prospective cohort. Ann King Edw Med Univ 23, 524–530. [Google Scholar]
- 46. Bater J, Lauer JM, Ghosh S et al. (2020) Predictors of low birth weight and preterm birth in rural Uganda: findings from a birth cohort study. PLoS One 15, e0235626. [DOI] [PMC free article] [PubMed] [Google Scholar]