Fig. 2. CART19/20 cells manufactured from naïve/memory T cells are enriched in memory phenotype.
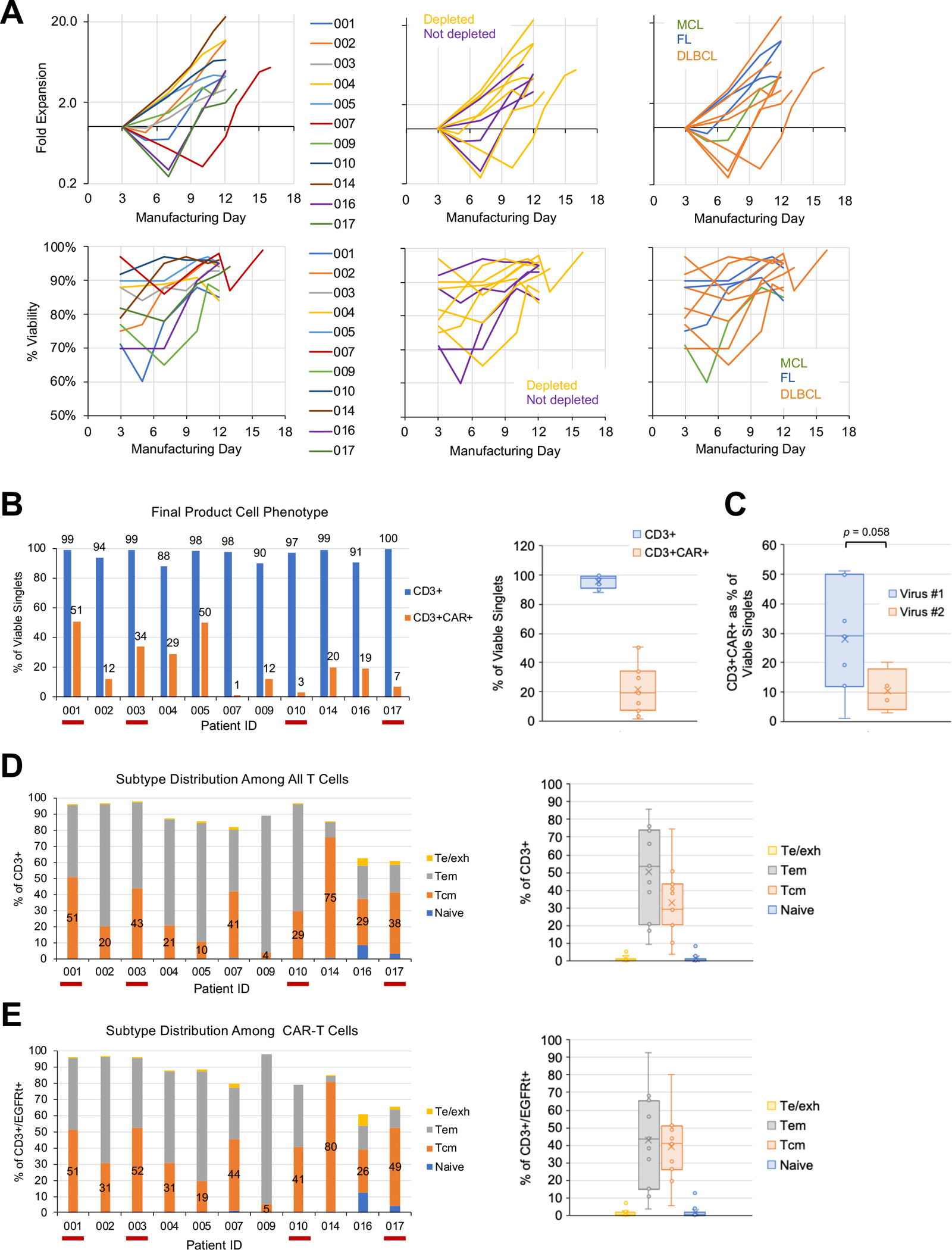
(A) Fold expansion (top) and viability (bottom) of cell product during ex vivo manufacturing. Cell counts were normalized to counts on the day of transduction (day 3). Data are shown with color coding by patient (left), by whether starting cell population underwent CD14/CD25 depletion (middle), and by disease indication (right). (B–E) Flow cytometry performed on cryopreserved cell aliquots post thaw to characterize (B) CD3+ purity and transduction efficiency of final cell product, (C) transduction efficiency grouped by batch of lentivirus used in manufacturing, (D) T-cell subtype distribution among all CD3+ T cells, and (E) T-cell subtype distribution among CAR-expressing T cells. Te/exh: effector/exhausted T cells, CD45RA+/CD45RO−/CD62L−; Tem: effector-memory T cells, CD45RA−/CD45RO+/CD62L−; Tcm: central-memory T cells: CD45RA−/CD45RO+/CD62L+; naïve: CD45RA+/CD45RO−/CD62L+. In panels B, D, and E, red underscoring of patient ID indicates products that did not undergo CD14/CD25 depletion.
