Fig. 5. CART19/20 cells exhibit sustained persistence and efficacy with strong safety.
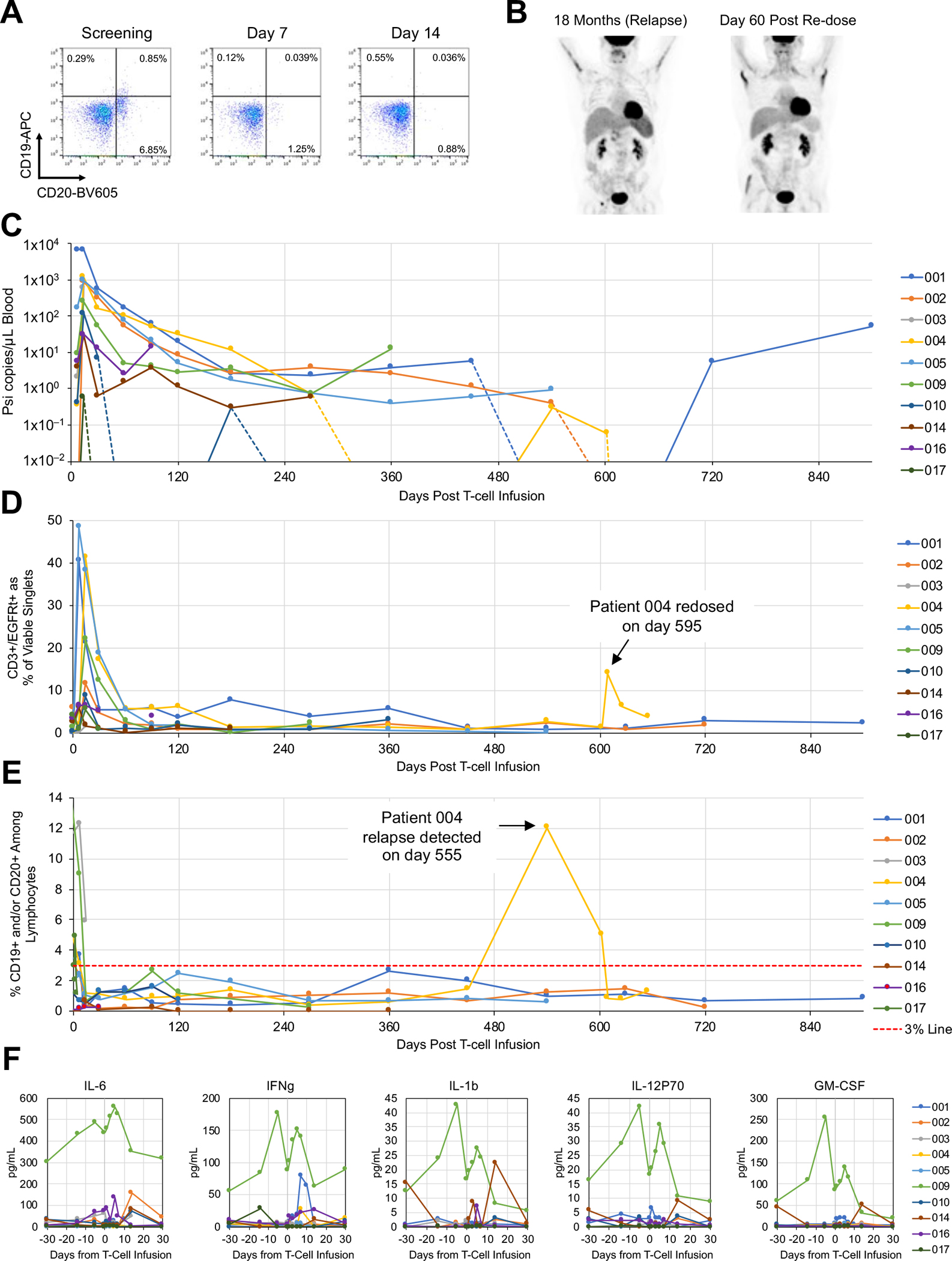
(A) Flow cytometry analysis on peripheral blood samples collected from Patient 004 at screening as well as 7- and 14-days post CART19/20 infusion. CD19 and CD20 surface staining results indicate the presence of CD20+CD19dim/– cells in Patient 004 prior to CART19/20 cell treatment. (B) PET scans of Patient 004 at the time of relapse and at 60 days post second dose of CART19/20 cells. (C) Presence of CAR transgene as quantified by droplet digital PCR. The psi signal integrated through lentiviral transduction was quantified. Inset shows zoomed-in data from the first 30 days post CART19/20 infusion. (D) Presence of CAR-expressing T cells among peripheral blood mononuclear cells (PBMCs) as quantified by flow cytometry. (E) Presence of CD19+ and/or CD20+ cells among lymphocytes as quantified by flow cytometry. A 3% line is shown to denote threshold for B-cell aplasia. (F) Serum cytokine levels as qualified by Luminex multiplex assay.
