Abstract
Exposure to certain per-and polyfluoroalkyl substances (PFAS) has been shown to be positively associated with total and/or low-density lipoprotein cholesterol. Examining this association in lipid lowering interventions may provide additional evidence linking PFAS to cardiovascular risk. We examined the relationship of 6 PFAS with cholesterol in a 6-month lifestyle-based intervention. We quantitated PFAS in 350 individuals at baseline and post intervention and examined associations of PFAS with cholesterol before and after intervention. Food frequency questionnaires and GIS analyses were used to investigate PFAS hotspots and possible exposure routes. Cholesterol significantly decreased following intervention and in parallel, PFOS, PFOA, PFHxS, and PFHpA significantly decreased. PFOS was positively correlated with total cholesterol only post-intervention. We observed that PFOS was distributed among both non-albumin and albumin lipoprotein fractions pre-intervention, but entirely in albumin fraction post-intervention. Our results indicate that lipid-lowering via lifestyle modification may impact on circulating levels or distribution of PFAS.
Keywords: PFAS, cardiovascular disease, cholesterol, PFOS, atherosclerosis
Introduction
Per-and polyfluoroalkyl substances (PFAS) are an emerging class of ubiquitous industrial chemicals that are measurable in the blood of most individuals due to their resilience to xenobiotic detoxification and elimination processes and consequent decades-long half-lives [1]. Specific PFAS, including perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) have been significantly associated to varying extents with increased circulating cholesterol and low-density lipoprotein (LDL) cholesterol—both clinical risk factors for cardiovascular disease—in multiple, epidemiological studies [2–6]. Although these associations have been validated in some preclinical models [7], there is still some disagreement of the causality of the relationship between PFAS and LDL cholesterol. Indeed, in some studies examining individuals with relatively high occupational exposures, PFAS levels were not associated with cholesterol [8, 9]. It is known that PFAS and other persistent halogenated pollutants can bind and be transported on plasma proteins including low-density lipoprotein (LDL) and albumin [10, 11]. These plasma proteins undergo temporal changes related to nutritional and organ disease status, which may be a confounder that could explain the inconsistencies in the observed relationship between PFAS and cholesterol [10, 11]. Studies show that PFAS bind primarily albumin in circulation, but these studies have been completed in healthy subjects with normal VLDL/LDL levels [12, 13]. To better understand the relationship among PFAS, circulating total cholesterol, and LDL cholesterol, we measured six environmentally relevant PFAS in the plasma of individuals undergoing a six-month lifestyle-based lipid lowering intervention and examined associations of circulating PFAS and cholesterol at baseline and at the end of the intervention program. Although PFAS was not a priori focus of the HeartHealth intervention, we hypothesized that associations between cholesterol and PFAS may differ pre and post lipid lowering, perhaps due to cellular level redistribution of lipids. The primary aim of this study was to determine if PFAS levels changed post intervention and if PFAS positively correlated with total and LDL cholesterol pre and/or post lifestyle intervention. A secondary aim utilized information collected from food-frequency questionnaires and GIS to identify possible exposure routes for PFAS, which may have changed due to the intervention, as it might offer insight into reasons for differences in associations between PFAS and cholesterol pre and post intervention. An exploratory aim of this study was to determine if lipid lowering would change the lipoprotein distribution of PFAS in humans enrolled in the intervention and confirmed using a mouse model of lipid lowering.
Methods
Subjects and clinical study design
This study was a sub-study of a patient-centered biobehavioral lifestyle intervention clinical trial (known as HeartHealth), which began enrollment in August 2013 and was completed in December 2016 (―HeartHealth in Rural Kentucky‖)[14]. The study was conducted using a pre-post intervention design with a wait-list control period. Outcomes were measured at baseline, after a 3-month wait list period with no intervention and then again in three months after the end of the intervention. Serum was obtained at baseline prior to data collection and following the post intervention at a mean of 6.4 months +/− 0.99 and range of 5–8 months after intervention was completed. All participants received the intervention thus no control group is available. Outcomes tested in this sub-study were PFAS, dietary intake and lipid profile. Eligibility criteria included residence in rural Kentucky, including Appalachia, absence of a primary care provider, and having at least 2 well-established CVD risk factors (e.g., hypertension, hypercholesterolemia, diabetes, sedentary lifestyle, overweight or obese). Participants were excluded if they were taking medications that might interfere with lipid metabolism or were known to already have CVD or chronic illnesses, such as kidney disease. Participants were recruited from rural areas of Kentucky based on Economic Research Service Rural-Urban Commuting Areas (RUCA) codes[15, 16]. Recruiters employed by the study were long-time members of the communities in which they recruited. We recruited (1) by advertising the study in local newspapers and on local radio and television stations; (2) using flyers at local areas such as churches, community centers, senior centers, gas stations, drug stores and public fairs; (3) by having local recruiters present the study at venues such as health fairs and other community engagement activities; and (4) by word of mouth. The intervention consisted of 12 biweekly group sessions, each focused on a topic relevant to CVD risk reduction. Participants were enrolled after providing informed signed consent. Full details of the intervention can be found in the supplemental materials.
Analytical method to quantitate PFAS
We measured PFAS by UHPLC electrospray ionization tandem mass spectrometry using our previously published analytical method [17] which monitors perfluorohexane sulfonate (PFHxS), perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoate (PFNA), perfluoroheptanoic acid (PFHpA), and perfluorobutanesulfonic acid (PFBS). In brief, PFAS were extracted from pre and post intervention plasma samples. 50 μl of plasma was extracted with methanol in 96 well Impact Protein Precipitation plates (Phenomenex). 13C labeled PFOA, PFOS, and PFNA were used as surrogates, and 13C labeled PFHpA was added as an internal standard. Each extraction plate contained blank samples and a range of quantitative quality controls and peak areas were compared to a standard curve to enable absolute quantitation of PFAS by stable isotope dilution methods. PFAS were measured using a Shimadzu HPLC coupled with an AB Sciex 6500-QTRAP hybrid linear ion trap triple quadrupole mass spectrometer operated in multiple reaction monitoring (MRM) mode. Samples were separated on an Atlantis T3, 3 um, 2.1 × 50 mm column and MRM transitions used for quantitation were as follows: 298.81/79.9 for PFBS, 462.94/418.8 for PFNA, 412.91/368.7 for PFOA, 398.89/79.9 for PFHxS, 362.91/318.7 for PFHpA, 498.88/79.9 for PFOS, 416.95/371.9 for 13C-PFOA, 502.93/79.8 for 13C-PFOS, 467.98/422.8 for 13C-PFNA, and 366.90/321.7 for 13C-PFHpA. Data was processed using AB Sciex Multiquant software, and PFAS concentrations (ng/mL) were determined for each sample. The limits of detection (LODs) were 0.04 (PFNA, PFOA), 0.05 ng/mL (PFBS, PFHpA), 0.2 ng/mL (PFOS, PFHxS). Mean spiked recoveries (n=10) of target analytes for 3 tier quality control (QC-low, QC-medium, QC-high) samples ranged from 70 – 127% with 2–14% relative standard deviation (RSD). The average spiked recoveries (n=10) of surrogates were 79 – 115% with 8 – 12% RSD for QC-low, 90 – 123% with 7 – 12% RSD for QC-medium, 90 – 123% with 7 – 12% RSD for QC-medium, 82 – 114% with 9 to 15% RSD for QC-high[17]. A total of 424 individuals were recruited for the study and a total of 350 pre and post samples were available for PFAS measurements.
Determination of dietary intake
Other than through contaminated drinking water, diet is a major exposure source for PFAS in the general public[18]. PFAS was not an initial focus of this HeartHealth study, thus unfortunately, no information related to source of water supply (municipal or well) or levels of PFAS in drinking water is available. Thus, it is plausible that any observed changes in circulating PFAS concentration may be due to changes in dietary habits promoted by the intervention. This phenomenon has been observed in studies of persistent organic pollutants [19]. Additionally, using water for irrigation or soil contaminated with PFAS may lead to possible exposures from fruits and vegetables [20]. The VioScreen™ online food-frequency questionnaire (FFQ; Viocare Inc., Princeton, New Jersey) was used to collect dietary intake patterns. This FFQ incorporates food images to assist participants when estimating portion sizes and solicits additional details while the participant indicates their responses. Food and nutrition information from the Nutrition Coordinating Center Food and Nutrient Database is used to generate dietary analyses. This FFQ is a reliable measure of energy-adjusted macronutrient intake when compared with 6, 24-hour dietary recalls conducted over 90 days and performs as well as paper-based FFQs[21]. All individuals provided dietary information, including consumption levels of meat, dairy, and fish (another source of PFAS).
Data from the FFQ was also used to generate cumulative dietary indices known as Healthy Eating Index (HEI)-2005 scores. The HEI-2005 is a measure of diet quality developed by the United States Department of Agriculture’s (USDA) Center for Nutrition Policy and Promotion to monitor adherence to 2005 national dietary recommendations [22]. Standards for components were based upon participant data from the 2001–2002 National Health and Nutrition Examination Survey (NHANES). Higher scores for each HEI component represent an intake closer to the standard, and maximum points are awarded for meeting the standard. Moderation components are reverse scored (i.e., greater intake corresponds with a lower score) due to the need to consume less of these items. The HEI-2005 total score is calculated by summing the component scores and can range from 0–100 with higher scores indicating better diet quality.
Statistical analyses
The data presented were generated using the following statistical methods: First, using a matched-pairs approach, we examined the change in total cholesterol, LDL cholesterol, body mass index (BMI) and six PFAS before and after intervention. As some PFAS variables were not normally distributed we also provide p-values from non-parametric Wilcoxon signed-rank test in a separate column. For each of the six individual PFAS we then calculated its correlation coefficient with total cholesterol or LDL cholesterol and a corresponding p-value in both pre-intervention and post-intervention samples. We also examined correlations between total and LDL cholesterol and multiple auxiliary variables (see Supplemental Table 1 row headings for variables of interest) to be used as covariates to include in subsequent multivariate models and determined corresponding p-values. These covariates were chosen because they may impact on lipid or PFAS levels independently. Since the only statistically significant correlation between any PFAS and cholesterol measurement was evident between PFOS and total cholesterol in post-intervention samples, we focused all subsequent analyses on this specific association. We then used linear regression modeling to assess the association of PFOS with total cholesterol when adjusted for other covariates that may independently modulate cholesterol levels or PFAS including BMI, age, race, gender, education level, and smoking history. Since all study participants investigated here underwent the lifestyle intervention, a control group comparison was not possible. Thus, we implemented the strategy of stratifying the study population into individuals that responded more or less favorably to the intervention as estimated by the participants’ post intervention decrease in total cholesterol. Participants who demonstrated a decrease of their total cholesterol greater than the study median (>6 mg/dL loss) were considered “responders”.
All PFAS variables were log transformed to reduce non-normality (one was added before taking the logarithm). A total of 424 individuals were recruited for the study and a total of 350 pre and post samples were available for PFAS measurements. For bivariate and linear regression analyses, individual PFAS below the LOD were enumerated with a value LOD/√2. JMP 12; SAS Institute Inc., Cary NC USA and Microsoft Excel (Microsoft Corporation, Redmond WA) environments were used for data analysis and visualization. A p-value of <0.05 was considered significant. Sample sizes for individual analyses are depicted in corresponding table legends.
Plasma cholesterol lowering in hyperlipidemic mice
The animal protocol was approved by the Lexington Veterans Affairs Medical Center (VAMC) Institutional Animal Care and Use Committee. 4 Male Ldlr −/− mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and fed a hyperlipidemic diet for 15 weeks ad libitum (Envigo Teklad; TD.88137). Mice were housed in a temperature- and light controlled-room (12 h light; 12 h dark) with water ad libitum. To promote cholesterol lowering, all mice were then transitioned to a lower fat chow diet for two weeks (Envigo Teklad 2018C). Blood was humanely collected by retro-orbital bleed prior to the diet transition and at study conclusion. EDTA:CTAD (1:5) was added to whole blood as an anticoagulant and was spun at 2000xg for 15 minutes to collect plasma. All mice were 16–21 weeks old prior to study commencement. Plasma samples were frozen in liquid nitrogen and stored at −80° C until extraction for PFAS measurements and cholesterol assay. Total free cholesterol was determined via a colorimetric assay (Pointe Scientific, #C7510). Plasma from this study was also fractionated into lipoprotein-associated compartments and measured for PFAS abundance as described below. Note that these animals were not exposed to exogenous PFAS and the levels of PFAS measured represent exposure from other sources, most likely diet and drinking water. As we were interested in mirroring the HeartHealth study as best as possible, all animals received the lipid lowering intervention via dietary change.
Plasma fractionation
100ul of matched plasma from mouse or human samples (from venipuncture) pre and post lipid lowering intervention were fractionated by size-exclusion chromatography using a calibrated Superose 6 (10/300GL; GE Healthcare) column attached to an Agilent 1100 HPLC to separate plasma into lipoprotein-associated fractions. The column was eluted with PBS and eluted at a flow rate of 0.5mL/min. Eluate was collected into a 96 deep-well plate using an Agilent 1200 (G1364B) collector as 24, 497μl fractions. All wells corresponding to specific fractions were combined based on previous western blot analysis (i.e., ApoA1, ApoB48/100, and Albumin are used to confirm correct fractions are collected) to generate fractions enriched in very low density lipoprotein (VLDL), LDL, high density lipoprotein (HDL), and albumin. Each total well volume corresponding to individual fractions were then extracted and analyzed to measure PFAS as above. Pre and post plasma samples were measured for 4 mice and a single human participant (due to sample volume availability).
Geospatial Analyses to identify possible environmental exposure sources
Data Acquisition and Processing
Large-scale PFAS exposure can occur through drinking water sources contaminated by proximity to PFAS-utilizing industries and can change over time (food and food contact materials are other important common exposure routes)[23]. The sources of PFAS exposure for rural Kentuckians are relatively unknown and no water samples were collected for the HeartHealth study. Using GIS approaches we aimed to identify correlations between PFAS and exposure sources pre and post intervention. The following publicly available spatial data sets containing variables potentially linked to sources of PFAS were downloaded: Perennial Streams and Associated Reservoirs, fast-food restaurants, EPA wastewater treatment sites, EPA Superfund sites, EPA PFOS facilities, and airport locations [24–27]. In the geospatial software, ArcGIS Pro 2.7, the Euclidean distance tool was used to generate a distance surface (raster) for the datasets across the entire study area. Zonal statistics were run on each variable to calculate average distances (i.e., distance to nearest airport) for each zip code generating the explanatory variables: WATER_DIST, EPA_WASTEWATER_DIST, FF2016 PER1000, EPA_SUPERFUND_DIST, NEI_AIRPORT_DIST. Average distance information for each item was stored in a database table. For these analyses we used PFAS levels specific to pre or post intervention and made correlations with zip codes for participants provided at baseline.
Geospatial Analysis in ArcGIS Pro
Using ArcGIS Pro 2.7, Optimized Hot Spot Analysis was conducted using the Getis-Ord Gi statistic for each PFAS group, correcting for multiple testing and spatial dependence, to identify statistically significant spatial clusters of high values (hot spots) and low values (cold spots) of blood levels for each of the six PFAS. To assess candidate spatial explanatory variables as potential PFAS exposure sources, separate analyses were run using the Exploratory Regression tool in ArcGIS Pro for PFHpA, PFHxS, PFNA, PFOA, and PFOS as the dependent variable. Age (MEDIAN_AGE), sex (POP_MALE, POP_FEMALE), and income (HH_INCOME_BENEFITS) data at the zip code level were obtained and included in the models along with the previously generated distance variables [28]. This tool uses Ordinary Least Squares (OLS) and Spatial Autocorrelation (Global Moran’s I).
Results
Biobehavioral lifestyle intervention is associated with decreases in circulating cholesterol and PFAS levels
The sample (Table 1) undergoing the holistic lifestyle-based intervention was predominately female (74.3%), obese (average BMI 31.6 + 6.8 kg/m2); and middle aged (52.4+ 13.1 years). Over 80% of participants were either overweight (30.9%) or obese (53.4%) by World Health Organization BMI category. Total cholesterol levels decreased from baseline levels of 197.6 ± 38.8 mg/dL to post-intervention levels of 190.9 ± 35.6 mg/dL (p<0.001); LDL cholesterol levels decreased from baseline levels of 115.0 ± 34.5 mg/dL to post-intervention levels of 112.6 ± 31.6 mg/dL (p<0.01); and HDL cholesterol was not significantly altered (46.8 ± 16.8 mg/dL to 45.7 ± 15.65 mg/dL, p=0.13). Body mass index declined marginally from baseline of 31.6 + 6.8 kg/m2 to a post-intervention level of 31.43 + 6.9 kg/m2 (p = 0.009).
Table 1.
Demographic information and PFAS pre and post intervention
| Total Sample (n=346)a | p-value (Paired T-tests) b | p-value (WSRT) c | |
|---|---|---|---|
|
| |||
| Age (Mean + SD) | 52.4+ 13.1 years | ||
| Sex (Count) | |||
| Male | 89 (25.7%) | ||
| Female | 257 (74.3%) | ||
| Race (Count) | |||
| African American | 10 (2.89%) | ||
| Caucasian | 330 (95.4%) | ||
| Other | 6 (1.73%) | ||
| Current County residency (Mean + SD) | 30.3+ 18.6 years | ||
| Smoking History | |||
| Current Smoker | 23 (6.65%) | ||
| Quit <1 year ago | 8 (2.31%) | ||
| Quit >1 year ago | 96 (27.7%) | ||
| Never Smoked | 219 (63.3%) | ||
| Total cholesterol (Mean + SD) | |||
| Pre Intervention | 197.6 + 38.8 mg/dL | p<0.001 | |
| Post Intervention | 190.9 + 35.6 mg/dL | ||
| LDL cholesterol (Mean + SD) | |||
| Pre Intervention | 115.0 + 34.5 mg/dL | p<0.01 | |
| Post Intervention | 112.6 + 31.6 mg/dL | ||
| HDL cholesterol (Mean + SD) | |||
| Pre Intervention | 46.8 + 16.8 mg/dL | p=0.13 | |
| Post Intervention | 45.7 + 15.5 mg/dL | ||
| Body mass index (Mean + SD) | |||
| Pre Intervention | 31.6 + 6.8 (Kg/m2) | p<0.01 | |
| Post Intervention | 31.4 + 6.9 (Kg/m2) | ||
| PFBS (Q1, Q3) | |||
| Pre Intervention | 0.04 (0.04, 0.04) ppb | p=0.190 | p=0.205 |
| Post Intervention | 0.04 (0.04, 0.04) ppb | ||
| PFOS (Q1, Q3) | |||
| Pre Intervention | 13.2 (7.19, 22.4) ppb | p<0.001 | p<0.001 |
| Post Intervention | 11.2 (6.16, 18.8) ppb | ||
| PFNA (Q1, Q3) | |||
| Pre Intervention | 1.04 (0.71, 1.59) ppb | p=0.241 | p=0.155 |
| Post Intervention | 1.06 (0.721, 1.42) ppb | ||
| PFOA (Q1, Q3) | |||
| Pre Intervention | 2.20 (1.44, 3.04) ppb | p<0.001 | p<0.001 |
| Post Intervention | 1.99 (1.35, 2.74) ppb | ||
| PFHxS (Q1, Q3) | |||
| Pre Intervention | 0.964 (0.386, 1.78) ppb | p=0.016 | p=0.006 |
| Post Intervention | 0.806 (0.384, 1.51) ppb | ||
| PFHpA (Q1, Q3) | |||
| Pre Intervention | 0.400 (0.04, 0.681) ppb | p=0.034 | p=0.051 |
| Post Intervention | 0.337 (0.04, 0.645) ppb | ||
Sample size may be less for some variables due to missing or suppressed data. Demographic information is based on pre-intervention responses.
For fluorinated compounds, matched pairs analysis was completed using log transformed data; log(x+1).
Matched pairs analysis was completed using Wilcoxon Signed Rank Test using non-log transformed data. PFBS= Perfluorobutanesulfonic acid; PFOS= Perfluorooctanesulfonic acid; PFNA= Perfluorononanoic acid; PFOA= Perfluorooctanoic acid; PFHxS= Perfluorohexane sulfonic acid; PFHpA= Perfluoroheptanoic acid. p<0.05 was considered statistically significant.
In parallel, PFOS levels decreased from 13.2 (Q1;719, Q3;22.4) ng/mL to 11.2 (Q1;6.16, Q3;18.8) ng/mL (p<0.001); PFOA decreased from 2.20 (Q1;1.44, Q3;3.04) ng/mL to 1.99 (1.35, 2.74) ng/mL (p<0.001); PFHxS decreased from 0.964 (0.386, 1.78) ng/mL to 0.806 (Q1;0.384, Q3;1.51) ng/mL (p=0.006); PFHpA decreased from 0.400 (Q1;0.04, Q3;0.681) ng/mL to 0.337 (Q1;0.04, Q3;0.645) ng/mL (p=0.034). There was no significant difference between PFNA (1.04 (Q1;0.710, Q3;1.59) ng/mL to 1.06 (Q1;0.721, Q3;1.42) ng/mL and PFBS levels were below the limit of detection. Geometric means and other summary statistics for PFAS measurements can be found in Supplemental Table 2.
PFOS is significantly positively associated with circulating cholesterol only after intervention
Table 2 shows the bivariate analyses correlating the six individual PFAS with either total cholesterol or LDL cholesterol in baseline or post-intervention samples. These PFAS were chosen to represent a more complex mixture than single congeners traditionally used in toxicity studies, but less complicated than a true environmental mixture of 10s-100s of PFAS. A combination of legacy, replacement, and emerging subtypes as well as both sulfonate and carboxylate types, the constituents of this mixture have been implicated in disorders of lipid metabolism and in cardiovascular disease, a primary focus of our lab. In baseline samples, no significant associations between any PFAS and total cholesterol were observed. Similarly, there was no significant association between any PFAS and LDL. However, in post-intervention samples, a weak significant positive association between PFOS and total cholesterol (correlation coefficient; 0.123, p-value; 0.024) was observed. PFOS is also significantly associated with LDL cholesterol in post-intervention samples (Pearson correlation coefficient; 0.129, p-value; 0.023).
Table 2.
Bivariate associations of PFAS and cholesterol levels
| PFAS | Correlation coefficient; p-value | |||
|---|---|---|---|---|
| Total Cholesterol | LDL Cholesterol | |||
| Pre-Intervention | ||||
| PFBS | 0.031 | 0.572 | 0.012 | 0.833 |
| PFOS | 0.020 | 0.711 | 0.063 | 0.267 |
| PFNA | −0.0078 | 0.886 | 0.004 | 0.949 |
| PFOA | −0.042 | 0.437 | −0.019 | 0.743 |
| PFHxS | −0.003 | 0.957 | −0.015 | 0.794 |
| PFHpA | −0.021 | 0.692 | −0.026 | 0.652 |
| Post-Intervention | ||||
| PFBS | −0.017 | 0.759 | −0.013 | 0.825 |
| PFOS | 0.123 | 0.024 | 0.129 | 0.023 |
| PFNA | −0.026 | 0.641 | −0.062 | 0.271 |
| PFOA | 0.038 | 0.487 | 0.021 | 0.712 |
| PFHxS | 0.001 | 0.984 | −0.026 | 0.649 |
| PFHpA | 0.022 | 0.694 | −0.006 | 0.919 |
LDL: Low-density lipoprotein; PFBS= Perfluorobutanesulfonic acid; PFOS= Perfluorooctanesulfonic acid; PFNA= Perfluorononanoic acid; PFOA= Perfluorooctanoic acid; PFHxS= Perfluorohexane sulfonic acid; PFHpA= Perfluoroheptanoic acid. Bold p-values represent p< 0.05; italicized p-values represent p< 0.10.
analysis was completed using log transformed data for PFAS; log(x+1). p<0.05 was considered statistically significant. N=333.
To begin to model the association between PFAS and cholesterol levels considering covariates that may independently alter cholesterol levels or impact on PFAS exposures, we examined the bivariate correlations of multiple auxiliary variables and total and LDL cholesterol in baseline and post-intervention samples. Examining covariates including sex, years residing in current county, education level, smoking history, age, race, BMI, and saturated fatty acid consumption (questionnaire), we determined that sex, residence time, and age were most predictive of total cholesterol levels (Supplemental Table 1). Focusing on the observed association between total cholesterol and PFOS in post intervention samples only, we then built a multivariate linear regression model that included the covariates BMI, age, education level, race, sex, and smoking history and determined that even after adjustment, PFOS was still significantly positively associated with total cholesterol in post-intervention samples (p-value; 0.013; Table 3). This overall model was significant (p < 0.001) and explained 8.0% of the variance in the outcome. Using the same covariates, PFOS was also a significant predictor of LDL cholesterol in post-intervention samples (p-value; 0.023; Supplemental Table 3). Adding income and employment status variables to the model had no effect on the PFOS association with cholesterol.
Table 3.
Multivariate model investigating adjusted associations of PFOS with total cholesterol post intervention
| Covariate | Estimated regression coefficient | Standard Error | p-value |
|---|---|---|---|
| Intercept | 167.3 | 21.7 | <0.001 |
| PFOSpost (log) | 5.65 | 2.27 | 0.013 |
| Sex | 17.3 | 4.52 | <0.001 |
| Age | 0.224 | 0.154 | 0.147 |
| Education level | 0.051 | 0.712 | 0.943 |
| Race | −2.94 | 3.79 | 0.438 |
| BMIpost | −0.172 | 0.277 | 0.535 |
| Smoking history | −2.17 | 2.36 | 0.358 |
| Years living in current county | 0.124 | 0.111 | 0.267 |
BMI: Body mass index.
The estimates above define a formula for the prediction of total cholesterol in subject’s post intervention.
For variable “sex”, 0=male. 1=female; For variable “Race”, 1=African American. 2=Caucasian. 3=Asian. 4=Hispanic/Latino. 5=American Indian/Alaskan Native. 6= Native Hawaiian/other Pacific. For variable “Smoking history”, 1=current smoker. 2=Stopped within 1 year. 3=stopped more than 1 year ago. 4=never smoked.
(n=333). R2= 0.08
The association between PFOS and total cholesterol is dependent on efficacy of the intervention
For participants who demonstrated a decrease of their total cholesterol greater than the study median (“responders”; >6 mg/dL), a significant positive association between PFOSpost and total cholesterolpost was observed (p-value; 0.002, n=163; Table 4). On the contrary, individuals in whom total cholesterol decreased less than the median of the total population (“non-responders”), no significant association between PFOSpost and total cholesterolpost was seen (p=0.944, n=172). A visual representation of these differences can be seen in Supplemental Figure 1. Thus, the association between cholesterol levels and PFAS was only seen in individuals with the greatest decrease in cholesterol levels post intervention. Additionally, we included a change in (delta) total cholesterol covariate to our regression model described above (Table 3B) and after this additional adjustment we still observed a significant positive association between PFOSpost and total cholesterolpost (p-value; 0.032, n=333). This overall model was also significant (p < 0.001) and explained 16% of the variance in the outcome.
Table 4.
Association of PFOS with total cholesterol is dependent on extent of lipid lowering
|
Non-responders Covariate |
Estimated regression coefficient | Standard Error | p-value |
| Intercept | 196.0 | 27.2 | <.001 |
| PFOSpost (log) | −0.226 | 3.20 | 0.944 |
| Sex | 17.4 | 6.21 | 0.006 |
| Age | 0.337 | 0.200 | 0.094 |
| Education level | −0.278 | 0.958 | 0.772 |
| Race | −1.39 | 4.63 | 0.764 |
| BMIpost | −0.326 | 0.359 | 0.366 |
| Smoking history | −4.83 | 3.43 | 0.160 |
| Years living in current county | 0.278 | 0.147 | 0.061 |
|
Responders Covariate |
Estimated regression coefficient | Standard Error | p-value |
| Intercept | 151 | 32.8 | <.001 |
| PFOSpost (log) | 9.57 | 3.02 | 0.002 |
| Sex | 14.2 | 6.14 | 0.022 |
| Age | 0.163 | 0.223 | 0.467 |
| Education level | 0.243 | 0.995 | 0.807 |
| Race | −5.38 | 5.94 | 0.367 |
| BMIpost | 0.009 | 0.405 | 0.983 |
| Smoking history | −1.38 | 3.07 | 0.653 |
| Years living in current county | 0.009 | 0.157 | 0.954 |
Responders lost >6 mg/dL total cholesterol (median loss). (n=171 non responders, n=162 responders).
BMI: Body mass index. PFOS= Perfluorooctanesulfonic acid
The estimates above define a formula for the prediction of total cholesterol in subject’s post intervention.
For variable “sex”, 0=male. 1=female; For variable “Race”, 1=African American. 2=Caucasian. 3=Asian. 4=Hispanic/Latino. 5=American Indian/Alaskan Native. 6= Native Hawaiian/other Pacific. For variable “Smoking history”, 1=current smoker. 2=Stopped within 1 year. 3=stopped more than 1 year ago. 4=never smoked.
Table 3B.
Multivariate model investigating adjusted associations of PFOS with total cholesterol post intervention including change in cholesterol post intervention
| Covariate | Estimated regression coefficient | Standard Error | p-value |
|---|---|---|---|
| Intercept | 170.8 | 20.7 | <.001 |
| PFOSpost (log) | 4.68 | 2.17 | 0.032 |
| Sex | 15.5 | 4.33 | <.001 |
| Age | 0.258 | 0.147 | 0.080 |
| Education level | 0.0861 | 0.680 | 0.8993 |
| Race | −3.56 | 3.62 | 0.326 |
| BMIpost | −0.110 | 0.265 | 0.679 |
| Smoking history | −2.47 | 2.25 | 0.273 |
| Years living in current county | 0.142 | 0.106 | 0.180 |
| Delta Total Cholesterol | −0.362 | 0.0639 | <.001 |
BMI: Body mass index.
The estimates above define a formula for the prediction of total cholesterol in subject’s post intervention.
For variable “sex”, 0=male. 1=female; For variable “Race”, 1=African American. 2=Caucasian. 3=Asian. 4=Hispanic/Latino. 5=American Indian/Alaskan Native. 6= Native Hawaiian/other Pacific. For variable “Smoking history”, 1=current smoker. 2=Stopped within 1 year. 3=stopped more than 1 year ago. 4=never smoked.
(n=333). R2= 0.16
Dietary habits correlate with PFAS exposure
Using food frequency questionnaires, we determined that the intervention significantly increased index scores for intake of total fruit, whole fruit, total vegetables, dark green and orange vegetables and legumes, whole grains and saturated fats while decreasing oils, sodium and total grains (Supplemental Table 4). There were no changes in possible PFAS sources including indices for Milk or Meat. The intervention did not have strong effects on fish consumption either, with only consumption of fried fish servings decreasing post intervention (Supplemental Table 5B). We next completed bivariate analyses between PFAS and these dietary indices using both pre and post intervention results (Table 5) and observed only a few significant correlations. PFBS was significantly negatively correlated with overall HEI-2005 scores pre intervention (Correlation coefficient; −.113, p=0.037), but showed no correlation post intervention. PFNA was significantly positively correlated with Healthy Eating Index scores pre intervention (Correlation coefficient; .146, p=0.007), but showed no correlation post intervention. PFHxS was significantly positively correlated with Healthy Eating Index scores pre intervention (Correlation coefficient; .109, p=0.043), and this correlation was strengthened post intervention (Correlation coefficient; .152, p=0.006). PFOS did not correlate with any dietary index pre intervention, but in post intervention, PFOS was significantly positively correlated with the meat and beans index (Correlation coefficient; .133, p=0.016). In general, significant correlations between individual PFAS and indices of fruit and vegetable intake were more common than with indices of fatty food intake (e.g., milk and oils). Fish consumption, an important exposure route for PFAS, was monitored as well, but only associations between PFNA and frequency of fish consumption was observed (Supplemental Table 5A). Finally, we added dietary intake measures of fiber, fat, cholesterol and protein to our multivariate model investigating adjusted associations of PFOS with total cholesterol post intervention and still observed significant positive associations with PFOS and cholesterol (Supplemental Table 6).
Table 5.
Bivariate associations of PFAS and Dietary Indices, N = 346
| Dietary Index | Correlation coefficient; p-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log_PFBS | Log_PFOS | Log_PFNA | Log_PFOA | Log_PFHxS | Log_PFHpA | |||||||
| r value | p | r value | p | r value | p | r value | p | r value | p | r value | p | |
| Pre intervention | ||||||||||||
| Total Healthy Eating Index | −.113* | 0.037 | 0.006 | 0.911 | .146** | 0.007 | 0.105 | 0.052 | .109* | 0.043 | 0.078 | 0.149 |
| Fruit | −0.087 | 0.108 | −0.067 | 0.214 | 0.021 | 0.693 | 0.003 | 0.949 | 0.044 | 0.422 | 0.070 | 0.198 |
| Non-Juice Fruit | −0.033 | 0.541 | −0.083 | 0.123 | 0.037 | 0.498 | −0.038 | 0.484 | 0.003 | 0.956 | 0.079 | 0.143 |
| Vegetables | −0.097 | 0.073 | 0.069 | 0.205 | .202** | <0.01 | .134* | 0.013 | 0.072 | 0.184 | .203** | <0.01 |
| Dark green, and orange vegetables and legumes | −.112* | 0.038 | 0.052 | 0.338 | .181** | <0.01 | .117* | 0.030 | .110* | 0.041 | .174** | 0.001 |
| Grains | 0.010 | 0.857 | 0.047 | 0.389 | −0.006 | 0.910 | 0.059 | 0.279 | 0.098 | 0.069 | −0.012 | 0.825 |
| Whole Grains | −0.058 | 0.284 | −0.002 | 0.966 | −0.025 | 0.650 | 0.013 | 0.806 | .133* | 0.014 | 0.016 | 0.771 |
| Milk | −0.008 | 0.889 | −0.074 | 0.173 | 0.002 | 0.972 | −0.012 | 0.827 | 0.021 | 0.697 | −0.058 | 0.281 |
| Meat/Beans | −.116* | 0.032 | 0.059 | 0.274 | 0.084 | 0.122 | 0.011 | 0.839 | 0.079 | 0.146 | 0.024 | 0.657 |
| Oils | −0.022 | 0.691 | 0.020 | 0.716 | 0.037 | 0.492 | 0.001 | 0.979 | −0.039 | 0.466 | 0.009 | 0.873 |
| Saturated Fat | −0.094 | 0.082 | −0.009 | 0.865 | 0.081 | 0.137 | .115* | 0.033 | 0.056 | 0.298 | 0.036 | 0.508 |
| Sodium | 0.083 | 0.124 | 0.003 | 0.961 | −0.089 | 0.099 | −0.029 | 0.591 | −0.036 | 0.501 | −.110* | 0.042 |
| Soluble fat, alcohol added sugars | −0.074 | 0.173 | 0.039 | 0.470 | .164** | 0.002 | 0.105 | 0.051 | 0.086 | 0.113 | 0.071 | 0.191 |
| Post intervention | ||||||||||||
| Total HEI | −0.003 | 0.959 | 0.022 | 0.687 | −0.009 | 0.868 | 0.011 | 0.841 | .152** | 0.006 | −0.005 | 0.925 |
| Fruit | −0.057 | 0.309 | −0.086 | 0.123 | −0.075 | 0.180 | −0.038 | 0.491 | 0.036 | 0.522 | 0.049 | 0.382 |
| Non-Juice Fruit | −0.063 | 0.256 | −0.049 | 0.377 | −0.042 | 0.448 | −0.026 | 0.638 | 0.031 | 0.580 | 0.058 | 0.300 |
| Vegetables | 0.048 | 0.389 | 0.049 | 0.380 | 0.041 | 0.467 | −0.013 | 0.811 | 0.059 | 0.291 | −0.018 | 0.754 |
| Dark green, orange vegetables and legumes | −0.003 | 0.956 | −0.030 | 0.595 | 0.027 | 0.633 | −0.018 | 0.745 | .113* | 0.042 | 0.006 | 0.915 |
| Grains | 0.024 | 0.667 | 0.000 | 0.994 | −0.025 | 0.650 | −0.021 | 0.701 | 0.090 | 0.108 | −0.052 | 0.354 |
| Whole Grains | 0.048 | 0.390 | 0.002 | 0.965 | −0.031 | 0.574 | −0.014 | 0.796 | 0.086 | 0.123 | 0.036 | 0.516 |
| Milk | 0.024 | 0.666 | −0.094 | 0.092 | −.119* | 0.033 | −0.053 | 0.344 | 0.028 | 0.618 | −0.023 | 0.682 |
| Meat/Beans | 0.104 | 0.061 | .133* | 0.016 | 0.061 | 0.274 | 0.084 | 0.132 | 0.069 | 0.216 | 0.027 | 0.627 |
| Oils | 0.030 | 0.591 | 0.019 | 0.727 | 0.039 | 0.489 | −0.034 | 0.544 | 0.000 | 0.994 | −0.007 | 0.899 |
| Saturated Fat | −0.048 | 0.391 | 0.092 | 0.098 | 0.053 | 0.345 | 0.071 | 0.203 | .137* | 0.014 | −0.030 | 0.591 |
| Sodium | −0.092 | 0.100 | −0.013 | 0.815 | 0.022 | 0.698 | 0.082 | 0.140 | −0.012 | 0.828 | 0.069 | 0.215 |
| Soluble fat, alcohol added sugars | 0.016 | 0.778 | 0.037 | 0.505 | −0.003 | 0.964 | −0.007 | 0.898 | .111* | 0.047 | −0.047 | 0.401 |
Bold p-values represent p< 0.05; italicized p-values represent p< 0.10.
analysis was completed using log transformed data for PFAS; log(x+1).
GIS Spatial analysis identifies PFAS hotspots and sources of exposure
Using an unbiased GIS approach, we identified PFAS “hotspots” at the zip code resolution for individuals taking part in the biobehavioral intervention. We utilized zip code information collected at baseline and correlated with PFAS levels pre or post intervention. Circulating PFHxS levels were significantly higher for individuals who resided around the Danville clinic location compared to the two other sites of recruitment, with especially elevated levels in areas including Berea, KY (Figure 1). A hotspot for plasma PFNA levels was identified in western Kentucky in the zip codes directly bordering Madisonville. A final hotspot was determined for circulating PFOA in the southwestern border with Tennessee in a zip code directly adjacent to the military base Fort Campbell. To identify exposure sources of PFAS for the individuals taking part in the intervention we used GIS spatial analyses to determine correlations between PFAS and distances from Superfund sites, wastewater districts, industrial sites related to PFAS use, water districts, airports, and fast-food restaurants. Table 6 shows the correlation coefficients and p-values for these analyses in both pre and post intervention samples. There were no significant correlations between PFOS and any distance measure in either pre or post intervention samples. PFNA showed an inverse (higher PFNA correlated with closer proximity) relationship with proximity to wastewater treatment plants both pre and post intervention. There were also positive significant correlations with PFNA and streams/reservoirs and fast-food restaurants only pre intervention. For PFOA, correlations resembled PFNA for streams and fast-food restaurants pre-intervention but the correlation between PFOA and streams was lost post intervention. Interestingly, there was also a near significant inverse correlation between PFOA and proximity to airports in pre and post samples (p=0.07). PFHxS showed only positive correlations, and these did not change post intervention (i.e., proximity to superfund sites and wastewater treatment facilities). Finally, PFHpA showed only one significant correlation which was an inverse relationship with proximity to PFAS industry which was evident only in post intervention samples (correlation coefficient; −0.337, p=0.013). We did not focus our analyses on PFBS due to this PFAS being primarily below limits of detection in our cohort.
Figure 1.
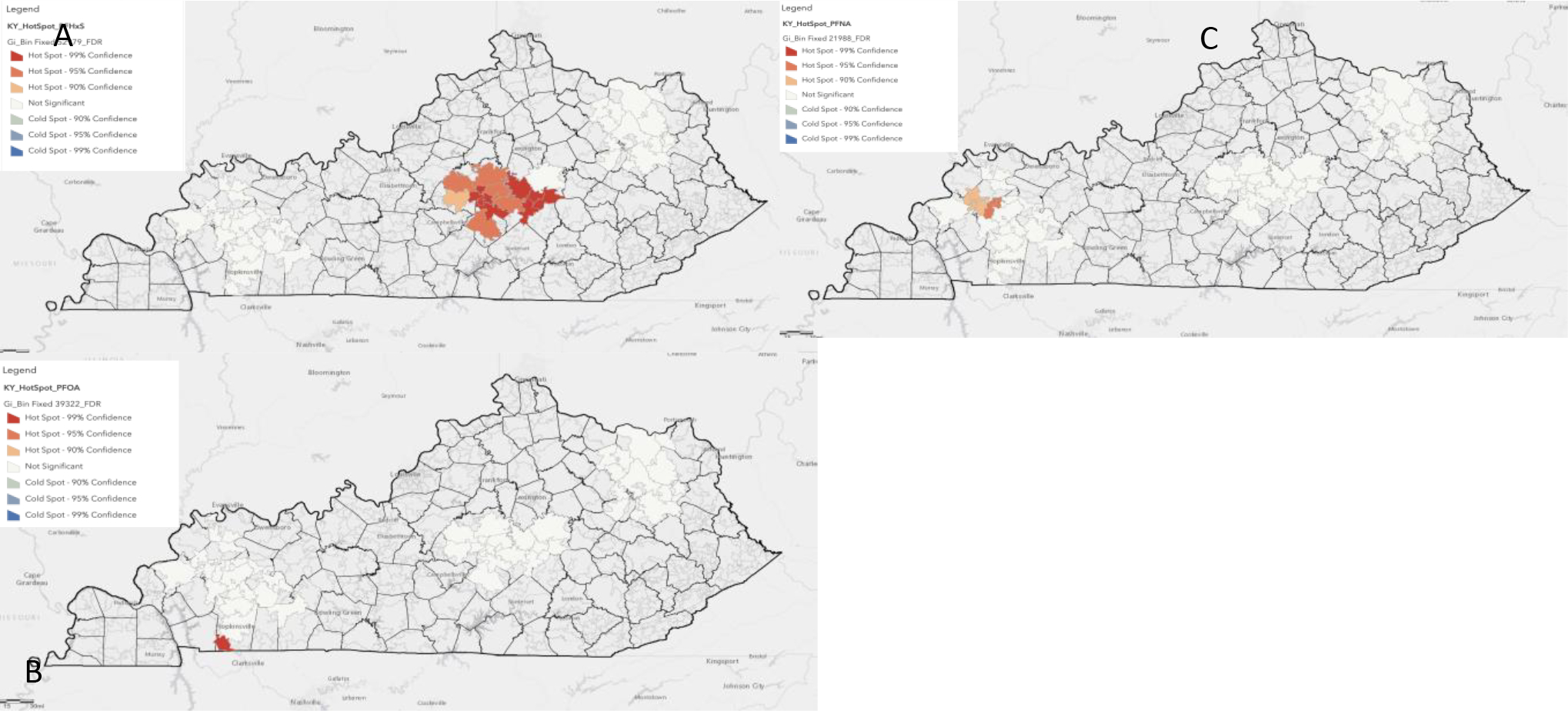
GIS spatial analysis identifies PFAS hotspots. A significant hotspot was identified for PFHxS in central Kentucky (A), PFOA in southwest KY nearby a military installation (B), and for PFNA in western Kentucky (C). Results are from zip code data collected at baseline and pre intervention circulating PFAS levels.
Table 6.
Bivariate associations of PFAS and distances to possible PFAS exposures
| PFAS | Correlation coefficient; p-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Superfund Sites | Wastewater treatment | PFAS Industry | Streams/Reservoirs | Airports | Fast-food restaurants | |||||||
| Pre-Intervention | ||||||||||||
| PFOS | 0.136 | 0.326 | −0.053 | 0.704 | −0.094 | 0.499 | 0.035 | 0.803 | −0.207 | 0.134 | 0.025 | 0.858 |
| PFNA | −0.108 | 0.438 | −0.295 | 0.030 | −0.039 | 0.782 | 0.269 | 0.049 | −0.131 | 0.346 | 0.275 | 0.044 |
| PFOA | −0.008 | 0.952 | 0.077 | 0.579 | 0.101 | 0.465 | 0.542 | <0.01 | −0.246 | 0.073 | 0.368 | 0.006 |
| PFHxS | 0.423 | 0.001 | 0.265 | 0.053 | 0.216 | 0.116 | −0.002 | 0.990 | −0.117 | 0.399 | −0.055 | 0.694 |
| PFHpA | 0.155 | 0.264 | −0.139 | 0.315 | 0.011 | 0.938 | −0.102 | 0.463 | −0.074 | 0.596 | −0.110 | 0.428 |
| Post-Intervention | ||||||||||||
| PFOS | 0.034 | 0.807 | 0.022 | 0.872 | −0.030 | 0.828 | −0.054 | 0.696 | −0.207 | 0.133 | 0.027 | 0.848 |
| PFNA | −0.229 | 0.095 | −0.282 | 0.039 | −0.099 | 0.478 | 0.026 | 0.850 | −0.098 | 0.483 | 0.208 | 0.130 |
| PFOA | −0.093 | 0.505 | −0.008 | 0.954 | 0.018 | 0.899 | 0.159 | 0.250 | −0.261 | 0.057 | 0.287 | 0.035 |
| PFHxS | 0.397 | 0.003 | 0.292 | 0.032 | 0.053 | 0.702 | −0.071 | 0.611 | 0.092 | 0.510 | −0.071 | 0.611 |
| PFHpA | 0.214 | 0.119 | −0.073 | 0.599 | −0.337 | 0.013 | −0.127 | 0.362 | −0.051 | 0.712 | −0.009 | 0.949 |
PFOS= Perfluorooctanesulfonic acid; PFNA= Perfluorononanoic acid; PFOA= Perfluorooctanoic acid; PFHxS= Perfluorohexane sulfonic acid; PFHpA= Perfluoroheptanoic acid. Bold p-values represent p< 0.05; italicized p-values represent p< 0.10.
Cholesterol lowering is associated with a redistribution of plasma PFOS between lipoprotein and albumin fractions
Since PFAS can bind and be transported by lipoproteins and it is well established that lipid lowering interventions may decrease VLDL, LDL, HDL, and albumin to differing extents, we fractionated baseline and post-intervention plasma from one of the “responders” in the clinical study (decreased cholesterol levels by 11 points) and subsequently extracted fractions corresponding to VLDL, LDL, HDL, and albumin for the six PFAS. Interestingly, in the baseline sample we observed PFOS (and all other PFAS as well) in both non-albumin fractions and the albumin fraction, but in the post-intervention sample all the quantifiable PFAS (97–99%) was observed in the albumin fraction (data not shown). Since large sample volumes necessary for FPLC analysis were not readily available from HeartHealth, we decided to follow up on this observation and explore the mechanism by using Ldl receptor (Ldlr) deficient hyperlipidemic mice where we can easily modulate cholesterol levels through diet. Taking advantage of the fact that mice in our animal facilities are regularly exposed to PFAS through their drinking water, cages, and/or diets, we fed Ldlr deficient mice a western style diet to raise circulating cholesterol and collected a baseline plasma sample for PFAS analysis. We then switched the mice to a regular chow diet for two weeks which effectively decreased circulating cholesterol levels from 579.0 mg/dL to 259.4 mg/dL (p<0.01, n=4; Figure 2A). As before, we fractionated plasma from baseline and post-intervention samples and extracted and analyzed for PFOS and the other fluorinated compounds. At baseline, 22.3% of PFOS was observed in the non-albumin fractions (VLDL, LDL, HDL) and 77.69% in the albumin fraction, but in post-intervention samples 100% of quantifiable PFOS was found in albumin fractions (Figure 2B). There was no statistically significant difference in total plasma PFOS concentration post two-week lipid lowering in this study (PFOSpre average; 0.70 ng/mL, PFOSpost average; 0.466 ng/mL; p=0.16). Unlike in the human fractionated sample, no other PFAS in mouse plasma followed this same shift (Figure 2C).
Figure 2.
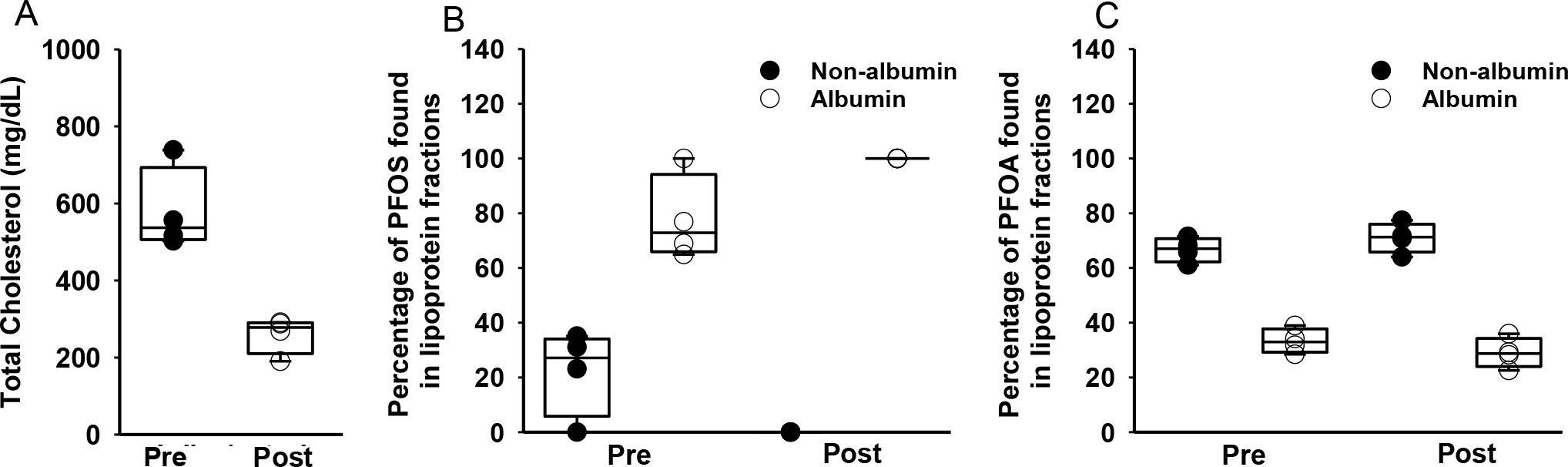
Cholesterol lowering in mice shifts PFAS abundance to albumin fraction. A) Cholesterol levels pre and post dietary modification. B) Male Ldlr deficient mice were fed a western style diet to increase circulating cholesterol and were then placed on a low-fat chow for 2 weeks to mirror lipid lowering intervention. The dietary intervention decreased circulating cholesterol levels from 579.0 mg/dL to 259.4 mg/dL (p<0.01, n=4) and shifted all quantifiable PFOS to the albumin fraction (77.69% to 100%). No change in PFOA was observed. (n=4 per group).
Discussion
It has been established by multiple epidemiological studies that exposure to legacy PFAS is positively associated with circulating cholesterol levels in humans[29]. Here we show, in a short-term lifestyle-based clinical intervention focused on lowering risk factors for cardiovascular disease, that circulating PFAS can decrease in parallel with the lowering of cholesterol levels, and that recent changes in circulating lipids are important variables for researchers to consider when examining associations between PFAS and cholesterol. Other longitudinal studies of much longer timeframe have also shown associations with individual PFAS and total cholesterol and have focused primarily on highly exposed populations[30–33]. A recent 10-year longitudinal study from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) (Uppsala, Sweden) also provides important results implicating more background levels of PFAS with cholesterol associations[34]. Similarly, a nested case-control study of older individuals from Sweden also reported significant associations between PFAS and cholesterol over time[35] Reports from prospective studies[3] are more rare, but also support the hypothesis that PFAS exposures are associated with increasing cholesterol levels. Regarding clinical intervention studies, ours is not the first to examine PFAS pre and post lipid lowering. For example, using samples from the Diabetes Prevention Program (ClinicalTrails.gov number, NCT00004992) which was a randomized controlled intense lifestyle intervention program, authors reported significant associations between baseline PFAS and hyperlipidemia, but associations were attenuated in participants receiving the intervention[36]. As our intervention was less severe and much shorter, it is hard to compare results between these studies, but both studies suggest controlling for changes in cholesterol over time may be important for future epidemiological studies.
Our observation that PFOS was only significantly associated with total cholesterol in post intervention samples was surprising and might be explained by multiple factors including changes in circulating cholesterol, other lipids, albumin, or by differences in acute exposures related to the intervention. However, with the current study design and lack of a control group, we can only postulate on this. Within this study we have begun to investigate some of these possibilities with our primary hypothesis related to the cholesterol lowering. However, as the lipid lowering was very modest here in relationship to the obvious changes in PFAS lipoprotein partitioning, alternative mechanisms may also be important. Our observations must be rigorously repeated in larger intervention studies with greater ranges of PFAS exposure It has been documented previously by Pizzurro et al., and others that PFAS bind to circulating albumin in rodents and in humans. More recently, these findings have been replicated in vitro with more than 90% of PFOS, PFOA, and PFHxS in serum bound to albumin and to LDL. Comparable values were seen in rodents and humans [37]. Most of this work has examined PFAS distributions in plasma from human subjects with relatively low circulating lipid levels, which is not the case with the cohort studied here. Unfortunately, albumin levels in the current study were not available at either timepoint in the human cohort. In our mouse model of diet dependent hyperlipidemia, we found that after lowering circulating lipids, PFOS was almost exclusively bound to albumin in serum, as opposed to PFOA, which did not have a significantly different distribution post-intervention. Although it is unclear why PFOS redistributed to albumin while PFOA did not, we hypothesize that the distinct functional groups, e.g., sulfonic acid group of PFOS versus carboxylic acid of PFOA, may contribute to distribution differences seen in our model. It will be critical for future studies to investigate albumin levels as a determinant of PFOS levels and distribution in plasma. In our mouse study we did not measure PFOS concentrations in the hyperlipidemic or chow diets, but we do not expect any slight differences to have impacted on our observed lipoprotein redistribution because PFOS has very long half-lives in rodents and humans[37]. Estimates for the half-life of PFOS in humans are 3.4 years or much longer and slightly less for rodents[38]. Additionally, in humans, 8-Carbon PFAS (PFOA) have a longer half-life compared to 4-Carbon PFAS, sulfonated PFAS have a longer half-life compared to carboxylated PFAS, and linear PFAS have a longer half-life compared to branched-chain PFAS. Because the half-life of PFAS in humans is so long, the impacts of our intervention are not explained by simple normal excretion. In fact, this study demonstrated a 15% decrease in PFOS levels from baseline in only approximately 6 months. Thus, our results support the hypothesis that the heart healthy intervention produced a more rapid decline in PFOS levels than can be accounted for by normal excretion.
It is also possible that the lifestyle intervention changed tissue distribution of PFAS, which may have impacted on circulating levels. This may be due to general weight loss or decreased adiposity of specific organs. Some evidence in humans implicates storage sites to include lung, kidney, liver, and others, but comparisons to blood levels are lacking[39]. Also, there have been some attempts to model body burden via physiologically based pharmacokinetic (PBPK) models, but more human tissue data is needed for these approaches [40]. Using tissue distribution results from rodent studies may be hindered by the observation that there may be species-specific differences. The current study did not include measurements of PFAS in tissues, however these levels could be determined in future studies via liver biopsies and compared to serum levels of PFAS to better establish how these halogenated pollutants distribute in humans. There is a large body of work investigating how reducing overall weight can impact serum levels of legacy persistent organic pollutants (POPs), but these reports may not be relatable to PFAS due to differences in lipophilicity. For example, Jansen, A et. al., analyzed the results of a handful of studies that focused on lipophilic POPs, mostly polychlorinated biphenyls, and they postulated that these types of halogenated pollutants are stored primarily in adipose tissue, thus rapid weight reduction (e.g., following bariatric surgery or diets), would increase circulating levels of the potentially harmful lipophilic pollutants. The results supported their hypothesis, with the average change in multiple POPs among studies ranging from a 2–4% increase in concentration for every kilogram of weight lost [41]. PFAS were either not measured or were not significantly correlated to weight loss in the selected studies. While this study may help guide our understanding of lipophilic pollutants in relation to weight and adipose tissue loss, it is important to note the dramatic alteration in enterohepatic circulation secondary to bariatric surgery. Absorption and metabolism of a multitude of nutrients and pollutants are changed due mostly to decreased surface area of the gastrointestinal system, an outcome specific to bariatric surgery that is not applicable in the current study. In addition, bariatric surgery has been shown to impact on expression of bile acid transporters[42], which have recently been show to also be transporters of diverse PFAS[43]. It is unknown if our 6 month intervention impacted on bile acids and or bile acid/PFAS transporter expression/activity. PFAS are bound to albumin and other lipoproteins in serum, so changes in adipose tissue volume may not have as big of an impact in PFAS levels as with PCBs and others [44]. In our study we did observe a significant, but mild, decrease in BMI post intervention, but including BMI in our regression models did not impact on the association with PFOS and total cholesterol. Future studies looking at PFAS pre and post bariatric surgery could confirm this difference with other legacy POPs as reported weight loss of those who undergo bariatric surgery can lose between 4.4 to 64.8 kg (9.7 to 142.9 lbs.), which is drastically more than the loss on average of approximately 3.8 kg (8.4 lbs.) in our study [41].
In addition to decreased circulating levels of PFAS, the intervention also demonstrated a shift in carrier protein binding that was reproduced in an experimental mouse model. Pre-intervention, PFAS was shown to be bound to proteins in an albumin faction as well as a non-albumin faction including LDL, HDL, and other lipoproteins. However, following the intervention, 100% of PFAS were bound to the albumin faction. We theorize that the intervention, which favors cardiovascular health, likely altered metabolism and possibly lipid storage. At baseline, this relatively unhealthy population had excess lipid storage, not only in adipose tissue, but also other organs. The intervention may have led to redistribution of PFAS and/or changes in albumin levels. The underlying mechanism for this result is still unclear, however, and will be expanded upon in future studies utilizing mouse models. A previous study using 326 samples from the POUNDS Lost Study (ClinicalTrials.gov number: NCT00072995) rigorously examined associations between PFAS and lipoprotein subspecies and reported that PFAS were positively associated with cholesterol, triglycerides, and apolipoproteins in IDL, LDL, and HDL containing atherogenic apoC-III[45]. PFAS were only measured in baseline samples and not 2 years post intervention so comparisons with this study are limited in relationship to changes lipoprotein partitioning post intervention. Although we hypothesize the shift is due to changes in lipid levels, the lifestyle intervention likely had impacts on physiology beyond cholesterol metabolism. Future studies comparing holistic lifestyle modifications versus pharmacological lipid lowering would be useful in identifying a mechanism of our observation as well as critically replicating this work in larger sample sizes.
Exposure routes of PFAS have been under investigation for years, with findings suggesting most exposure to PFAS is through diet and water intake. Previously, PFOA has been found in prominent levels in butter, olive oil, salmon, and catfish, with highest PFOA dietary intake from meat [46]. More recently in 2019, Kedikoglou et. al,. demonstrated that fish had the highest levels of PFAS, specifically PFOA and PFOS [47]. In addition, PFOA was found in high levels in chicken and PFOS was found in high levels in eggs. Overall, PFOS demonstrated the highest bioaccumulation potential. The current study, however, showed significant associations between PFNA, PFOA, PFHxS, and PFHpA and dark green vegetables. There have been some reports of PFAS contaminating fruits and vegetables, but these events are likely limited to areas of groundwater or soil contamination[20]. Future studies measuring PFAS in drinking water and soils of participants of the HeartHealth intervention are warranted. PFOS showed no significant correlations between any individual food items or cumulative index pre or post intervention, so it is unlikely that alterations in diet account for the change in PFOS distribution. However, some PFAS did show significant associations between food items pre and post. Of note, pre-intervention PFOA was significantly positively associated with vegetables and saturated fats, but PFOA had no significant correlations post-intervention. Additionally, PFNA pre-intervention was positively associated with total healthy eating index, vegetables, and added sugars, while post-intervention PFNA was negatively associated with milk. Fish are known to be a significant source of PFAS exposure while also being associated with lower LDL cholesterol levels. To capture the impact of fish consumption on PFAS, we relied on questions included in the dietary survey that focused on fried and nonfried fish variables. Additionally, EPA and DHA, possible biomarkers of fish intake, were estimated by the FFQ responses. Associations between fish intake and PFAS, as well as cholesterol levels, were for the most part absent, which may be due to the observed low level of fish consumption in the population. Future questionnaires should delineate between locally caught fish and store-bought fish. Overall, there were no remarkable correlations between PFAS and food items that could explain the change in association between PFOS and cholesterol observed only post-intervention. In addition, the lifestyle-based intervention may have impacted on circulating PFAS concentrations by modulating fiber intake which has recently been implicated as means to decrease PFAS levels. For example, Dzierlenga et. al., reported that diets with increased fiber were associated with decreased PFOA, PFOS, and PFNA due to increased gastrointestinal excretion[48]. Fiber is known to reduce the absorption of bile acids by trapping them in the gut lumen. As bile acids are imperative for the absorption of large fatty acids and cholesterol, dietary fiber is linked to lower circulating levels of cholesterol. As stated previously, cholesterol levels have been repeatedly shown to be associated with circulating PFAS levels. Therefore, dietary fiber intake may be an important modulating factor of this association. Clinically, there is some evidence that impacting enterohepatic circulation can decrease circulating cholesterol [49] and PFAS levels. Bile acid sequestrants, specifically cholestyramine, have been shown to have an impact on reducing PFAS levels via elimination in feces. As early as 1984, a study in rat models by Johnson et al., demonstrated that when treated with cholestyramine, the experimental group excreted almost 10 times as much PFOA and PFOS in their feces than the control group [50]. Additionally, a case report in 2010 by Genuis et al., found that in a man with elevated levels of serum PFAS, cholestyramine treatment led to increased PFAS detected in stool and decreased PFAS in serum with continued use [51]. Limited data is available regarding this association between bile acid sequestrants and lower serum PFAS levels, but it offers another route of investigation for future studies. While bile acid homeostasis was not measured in our cohort, it could be interesting to see if the healthy heart intervention led to any changes in bile acid metabolism and cholesterol recycling. We do not believe medication use had any impact on our findings as individuals on lipid lowering therapies were excluded from this study.
Proximity to environmental sources is another important determinant of human PFAS exposure. We used GIS mapping analysis to explore the relationship between site of residence and PFAS exposure with the goal of identifying PFAS “hotspots.” GIS mapping has been implemented by many researchers to locate hotspots of pollutants; chronic diseases; bacterial, viral, and parasitic infections; along with many other variables to determine their spatial distribution. PFAS hotspots were identified in urbanized areas and near a military base, and residential proximity to wastewater plants, airports, and PFAS-utilizing industries were predictive of higher PFAS concentrations. There were no significant correlations with PFOS either pre or post intervention for any of the proximity measures investigated. Previous studies utilizing GIS mapping have identified multiple types of PFAS exposure sources, most prominently industrial sites and aqueous film-forming foam (AFFF)-impacted areas [52]. Specifically, Hu et al., found elevated, but of variable significance, concentrations of PFBS, PFHxS, PFOS, PFOA, PFNA, and PFHpA in drinking water around major industrial sites, military fire training areas, AFFF-certified airports, and wastewater treatment plants [53]. Another study compared surface and ground water PFAS concentrations, finding that concentrations increased with increasing depth. This study further reported that PFAS concentrations were higher around manufacturing sites that produced fluoropolymers [54]. A limitation of our results is that we utilized zip code level data instead of longitude and latitude information and did not have access to PFAS concentrations in wells or municipal water systems.
Conclusion
In conclusion, this study adds to the growing body of literature regarding our knowledge of per- and polyfluoroalkyl substances and their associations with increased circulating cholesterol, a critical mediator of cardiovascular disease. The levels of PFAS observed, except for PFOS, were comparable to those measured in NHANES for the corresponding years of 2015–2016, (i.e., median PFHxS: 1.2 ng/mL, PFOA: 1.5 ng/mL, PFNA: 0.6 ng/mL, and PFOS: 3.2 ng/mL), allowing our findings to be more generalizable to average adults in the United States [55]. This cohort also had clinically significant cholesterol levels at baseline, with an average total cholesterol of 197.6. According to the CDC, the average total cholesterol in American adults is 191 mg/dL and approximately 36% of US adults have cholesterol levels around 200 mg/dL or higher. This cohort therefore shows similarities to the average US adult while also creating a large gradient of change during the healthy heart intervention over which to examine how PFAS levels were altered [56]. Additionally, this study incorporates repeated measures of PFAS levels in a controlled, clinical study environment, which adds more context than a single timepoint measurement. There are important limitations to this study as well. The study population is homogenous, with a predominance of older, Caucasian, obese females, which limits the extrapolation potential of the results. In comparison to other epidemiological studies the sample size is modest, which is especially important for studies like this one where the distribution of PFAS exposures is less diverse. Likewise, although baseline cholesterol levels were clinically determined to be high, cohorts with more severe hypercholesterolemia and subjected to more aggressive pharmacological management would likely show much larger reductions in circulating cholesterol post-intervention. Furthermore, this study did not include participants with other chronic diseases, specifically kidney disease or liver disease. As functioning renal and hepatic systems are imperative in maintaining circulating albumin, derangements in these systems could lead to a different shift in PFAS binding away from albumin. While our population had risk factors for kidney disease (hypertension, diabetes), we did not enroll anyone with known kidney or other chronic diseases. As multiple studies have shown that kidney disease/dysfunction can decrease serum PFAS, future intervention studies should monitor changes in eGFR and albumin [57, 58]. Additionally, this study did not rigorously examine fish intake which is an important exposure source of PFAS as well as associated with lower LDL/total cholesterol. Finally, as this study had no control group, it is not a true randomized controlled trial, so it is not possible to infer a causal relationship between the intervention components and alterations in PFAS. Specifically, the lack of a control group proposes a challenge in interpreting the shift in PFAS lipid and protein binding. Measuring the fractional distribution of PFAS in a control group without intervention would yield important information about temporal changes in PFAS binding unrelated to dietary and modest lifestyle changes.
Supplementary Material
Highlights.
PFAS were measured in serum of 350 individuals undergoing a lipid lowering intervention
The HeartHealth intervention led to decreased circulating cholesterol and PFAS
PFOS significantly positively associated with cholesterol only post intervention
Lipid lowering in humans and mice modulates lipoprotein distribution of PFAS
Acknowledgements:
We thank Dr. Erin Bunting and Bob Goodwin (Michigan State University) for their valuable discussion on spatial statistics and GIS analysis.
Funding:
This research was supported in part by the National Institute of Environmental Health Sciences [P30ES020957, P30ES026529, R00ES028734], the Office of the Vice President for Research at Wayne State University, Health Resources and Services Administration, D1ARH20134, and used resources at the Lexington, KY VA medical center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Stephanie Morgan: Writing, Software M. Abdul Mottaleb.: Data curation, Methodology, Validation. Maria P. Kraemer: Investigation, Writing-Reviewing and Editing. Debra K. Moser: Supervision, Software, Data curation, Funding: Jessica Worley: Software. Andrew J Morris: Conceptualization, Reviewing and Editing, Supervision. Michael C Petriello: Conceptualization, Writing-Reviewing and Editing, Funding, Supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fu J, et al. , Occurrence, temporal trends, and half-lives of perfluoroalkyl acids (PFAAs) in occupational workers in China. Sci Rep, 2016. 6: p. 38039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eriksen KT, et al. , Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS One, 2013. 8(2): p. e56969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitz-Simon N, et al. , Reductions in serum lipids with a 4-year decline in serum perfluorooctanoic acid and perfluorooctanesulfonic acid. Epidemiology, 2013. 24(4): p. 569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geiger SD, et al. , The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere, 2014. 98: p. 78–83. [DOI] [PubMed] [Google Scholar]
- 5.Jain RB and Ducatman A, Associations between lipid/lipoprotein levels and perfluoroalkyl substances among US children aged 6–11 years. Environ Pollut, 2018. 243(Pt A): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 6.Kang H, et al. , Perfluoroalkyl acids in serum of Korean children: Occurrences, related sources, and associated health outcomes. Sci Total Environ, 2018. 645: p. 958–965. [DOI] [PubMed] [Google Scholar]
- 7.Rebholz SL, et al. , Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice. Toxicol Rep, 2016. 3: p. 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen GW and Zobel LR, Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int Arch Occup Environ Health, 2007. 81(2): p. 231–46. [DOI] [PubMed] [Google Scholar]
- 9.Rotander A, et al. , Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF). Environ Int, 2015. 82: p. 28–34. [DOI] [PubMed] [Google Scholar]
- 10.Han X, et al. , Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem Res Toxicol, 2003. 16(6): p. 775–81. [DOI] [PubMed] [Google Scholar]
- 11.Salvalaglio M, Muscionico I, and Cavallotti C, Determination of energies and sites of binding of PFOA and PFOS to human serum albumin. J Phys Chem B, 2010. 114(46): p. 14860–74. [DOI] [PubMed] [Google Scholar]
- 12.Forsthuber M, et al. , Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma. Environ Int, 2020. 137: p. 105324. [DOI] [PubMed] [Google Scholar]
- 13.Butenhoff JL, et al. , Distribution of perfluorooctanesulfonate and perfluorooctanoate into human plasma lipoprotein fractions. Toxicol Lett, 2012. 210(3): p. 360–5. [DOI] [PubMed] [Google Scholar]
- 14.Mudd-Martin G, et al. , Rural Appalachian perspectives on heart health: social ecological contexts. Am J Health Behav, 2014. 38(1): p. 134–43. [DOI] [PubMed] [Google Scholar]
- 15.Kulshreshtha A, et al. , Urban-rural differences in coronary heart disease mortality in the United States: 1999–2009. Public Health Rep, 2014. 129(1): p. 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamm L and Hutchison L, Rural health priorities in America: where you stand depends on where you sit. J Rural Health, 2003. 19(3): p. 209–13. [DOI] [PubMed] [Google Scholar]
- 17.Petriello MC, et al. , Serum concentrations of legacy and emerging per- and polyfluoroalkyl substances in the Anniston Community Health Surveys (ACHS I and ACHS II). Environ Int, 2022. 158: p. 106907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth K, et al. , Diet as an Exposure Source and Mediator of Per- and Polyfluoroalkyl Substance (PFAS) Toxicity. Frontiers in Toxicology, 2020. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y and Fang M, Nutritional and Environmental Contaminant Exposure: A Tale of Two Co-Existing Factors for Disease Risks. Environ Sci Technol, 2020. 54(23): p. 14793–14796. [DOI] [PubMed] [Google Scholar]
- 20.Brown JB, et al. , Assessing Human Health Risks from Per- and Polyfluoroalkyl Substance (PFAS)-Impacted Vegetable Consumption: A Tiered Modeling Approach. Environ Sci Technol, 2020. 54(23): p. 15202–15214. [DOI] [PubMed] [Google Scholar]
- 21.Kristal AR, et al. , Evaluation of web-based, self-administered, graphical food frequency questionnaire. J Acad Nutr Diet, 2014. 114(4): p. 613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guenther PM, et al. , Evaluation of the Healthy Eating Index-2005. J Am Diet Assoc, 2008. 108(11): p. 1854–64. [DOI] [PubMed] [Google Scholar]
- 23.Kurwadkar S, et al. , Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci Total Environ, 2022. 809: p. 151003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Survey, U.S.G., National Hydrography Dataset (ver. USGS National Hydrography Dataset Best Resolution (NHD) for Hydrologic Unit (HU) 4 – 2001 (published 20191002). https://www.usgs.gov/core-science-systems/ngp/national-hydrography/access-national-hydrography-products, 2019.
- 25.Federal Aviation Administration, A.I.S., Airports. 2021.
- 26.Agency, U.S.E.P., TRI Explorer (2019 Updated Dataset). 2021.
- 27.(ERS), E.R.S., Food Access Research Atlas. U.S. Department of Agriculture (USDA), 2020. [Google Scholar]
- 28.Survey, U.S.C.B.A.C., American Community Survey 1-Year Estimates. 2018.
- 29.Andersen ME, et al. , Why is elevation of serum cholesterol associated with exposure to perfluoroalkyl substances (PFAS) in humans? A workshop report on potential mechanisms. Toxicology, 2021. 459: p. 152845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakr CJ, et al. , Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J Occup Environ Med, 2007. 49(8): p. 872–9. [DOI] [PubMed] [Google Scholar]
- 31.Costa G, Sartori S, and Consonni D, Thirty years of medical surveillance in perfluooctanoic acid production workers. J Occup Environ Med, 2009. 51(3): p. 364–72. [DOI] [PubMed] [Google Scholar]
- 32.Olsen GW, et al. , Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J Occup Environ Med, 2003. 45(3): p. 260–70. [DOI] [PubMed] [Google Scholar]
- 33.Winquist A and Steenland K, Modeled PFOA exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts. Environ Health Perspect, 2014. 122(12): p. 1299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunder L, et al. , Changes in plasma levels of per- and polyfluoroalkyl substances (PFAS) are associated with changes in plasma lipids - A longitudinal study over 10 years. Environ Res, 2022. 211: p. 112903. [DOI] [PubMed] [Google Scholar]
- 35.Schillemans T, et al. , Per- and Polyfluoroalkyl Substances and Risk of Myocardial Infarction and Stroke: A Nested Case-Control Study in Sweden. Environ Health Perspect, 2022. 130(3): p. 37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin PD, et al. , Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults-longitudinal analysis of the diabetes prevention program outcomes study. Environ Int, 2019. 129: p. 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pizzurro DM, et al. , Interspecies differences in perfluoroalkyl substances (PFAS) toxicokinetics and application to health-based criteria. Regul Toxicol Pharmacol, 2019. 106: p. 239–250. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, et al. , Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med, 2018. 75(1): p. 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez F, et al. , Accumulation of perfluoroalkyl substances in human tissues. Environ Int, 2013. 59: p. 354–62. [DOI] [PubMed] [Google Scholar]
- 40.Fabrega F, et al. , PBPK modeling for PFOS and PFOA: validation with human experimental data. Toxicol Lett, 2014. 230(2): p. 244–51. [DOI] [PubMed] [Google Scholar]
- 41.Jansen A, et al. , Increased blood levels of persistent organic pollutants (POP) in obese individuals after weight loss-A review. J Toxicol Environ Health B Crit Rev, 2017. 20(1): p. 22–37. [DOI] [PubMed] [Google Scholar]
- 42.KITAHARA Y, et al. , Enterohepatic Circulation of Bile Acids Is Activated by Bariatric Surgery before Weight Loss Becomes Apparent. Diabetes, 2018. 67(Supplement_1). [Google Scholar]
- 43.Zhao W, et al. , Organic Anion Transporting Polypeptides Contribute to the Disposition of Perfluoroalkyl Acids in Humans and Rats. Toxicol Sci, 2017. 156(1): p. 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bangma J, et al. , Understanding the dynamics of physiological changes, protein expression, and PFAS in wildlife. Environ Int, 2022. 159: p. 107037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu G, et al. , Associations of Perfluoroalkyl substances with blood lipids and Apolipoproteins in lipoprotein subspecies: the POUNDS-lost study. Environ Health, 2020. 19(1): p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schecter A, et al. , Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ Health Perspect, 2010. 118(6): p. 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kedikoglou K, et al. , Preliminary assessment of general population exposure to perfluoroalkyl substances through diet in Greece. Environ Res, 2019. 177: p. 108617. [DOI] [PubMed] [Google Scholar]
- 48.Dzierlenga MW, Keast DR, and Longnecker MP, The concentration of several perfluoroalkyl acids in serum appears to be reduced by dietary fiber. Environ Int, 2021. 146: p. 106292. [DOI] [PubMed] [Google Scholar]
- 49.Ewang-Emukowhate M and Wierzbicki AS, Lipid-lowering agents. J Cardiovasc Pharmacol Ther, 2013. 18(5): p. 401–11. [DOI] [PubMed] [Google Scholar]
- 50.Johnson JD, Gibson SJ, and Ober RE, Cholestyramine-enhanced fecal elimination of carbon-14 in rats after administration of ammonium [14C]perfluorooctanoate or potassium [14C]perfluorooctanesulfonate. Fundam Appl Toxicol, 1984. 4(6): p. 972–6. [DOI] [PubMed] [Google Scholar]
- 51.Genuis SJ, et al. , Human detoxification of perfluorinated compounds. Public Health, 2010. 124(7): p. 367–75. [DOI] [PubMed] [Google Scholar]
- 52.Nickerson A, et al. , Spatial Trends of Anionic, Zwitterionic, and Cationic PFASs at an AFFF-Impacted Site. Environ Sci Technol, 2021. 55(1): p. 313–323. [DOI] [PubMed] [Google Scholar]
- 53.Hu XC, et al. , Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ Sci Technol Lett, 2016. 3(10): p. 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robuck AR, et al. , Legacy and Novel Per- and Polyfluoroalkyl Substances in Juvenile Seabirds from the U.S. Atlantic Coast. Environ Sci Technol, 2020. 54(20): p. 12938–12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kotlarz N, et al. , Measurement of Novel, Drinking Water-Associated PFAS in Blood from Adults and Children in Wilmington, North Carolina. Environ Health Perspect, 2020. 128(7): p. 77005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Statistics N.C.f.H., Cholesterol. Centers for Disease Control and Prevention, 2021. [Google Scholar]
- 57.Jain RB and Ducatman A, Perfluoroalkyl acids serum concentrations and their relationship to biomarkers of renal failure: Serum and urine albumin, creatinine, and albumin creatinine ratios across the spectrum of glomerular function among US adults. Environ Res, 2019. 174: p. 143–151. [DOI] [PubMed] [Google Scholar]
- 58.Moon J, Perfluoroalkyl substances (PFASs) exposure and kidney damage: Causal interpretation using the US 2003–2018 National Health and Nutrition Examination Survey (NHANES) datasets. Environ Pollut, 2021. 288: p. 117707. [DOI] [PubMed] [Google Scholar]
- 59.Ford ES, et al. , Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med, 2007. 356(23): p. 2388–98. [DOI] [PubMed] [Google Scholar]
- 60.Maruthur NM, Wang NY, and Appel LJ, Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation, 2009. 119(15): p. 2026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rugg SS, Bailey AL, and Browning SR, Preventing cardiovascular disease in Kentucky: epidemiology, trends, and strategies for the future. J Ky Med Assoc, 2008. 106(4): p. 149–61; quiz 149, 162–3. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


