Fig. 2 |. New T cell targets in the HNSCC TME.
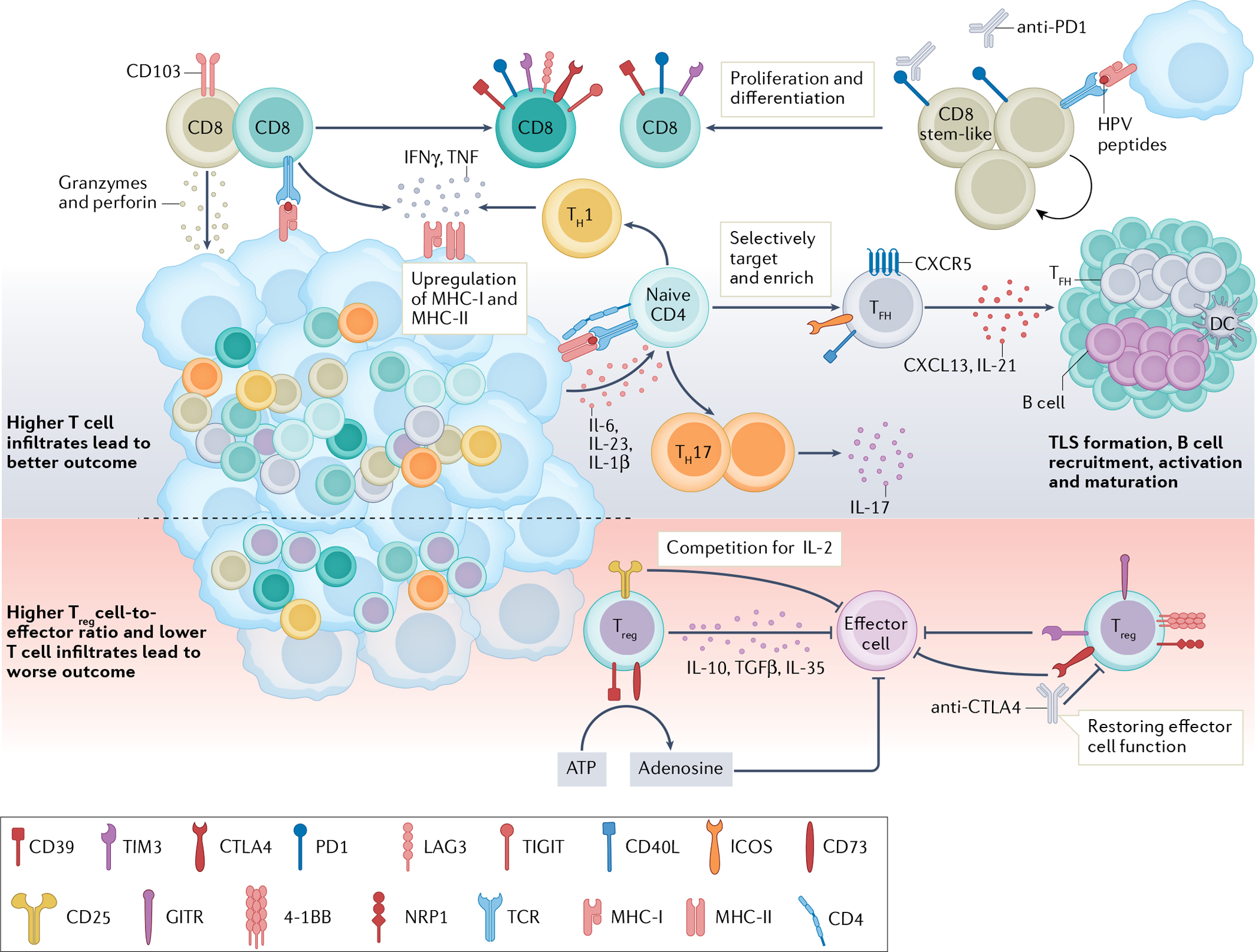
T cells are important in antitumour immunity, and human papillomavirus (HPV)-positive tumours generally show higher T cell infiltration and are associated with better outcomes than HPV-negative tumours32–34. CD8+ T cells can directly lyse target cells by releasing granzymes and perforin in an antigen-directed manner. Chronic engagement with tumour antigens leads to a dysfunctional state characterized by high expression of immune-checkpoint receptors (PD1, T cell immunoglobulin and mucin domain 3 (TIM3), lymphocyte-activation protein 3 (LAG3) and CTLA4)36–39. An HPV16-specific CD8+PD1+ T cell population in HPV-positive tumours contains a stem-like CD8+ T cell subset. These cells are capable of proliferating and differentiating into effector cells upon HPV peptide stimulation and represent a population of future therapeutic targets28. CD4+ T lymphocytes recognize major histocompatibility complex (MHC) class II antigens and differentiate into different subtypes upon antigen stimulation. A higher number of T helper 1 (TH1) and TH17 cells are found in HPV-positive tumours than in HPV-negative tumours48,49. TH1 cells release IFNγ and TNF and promote MHC class I and II upregulation on cancer cells, facilitating tumour elimination43,44. IL-17-releasing TH17 cells are induced by IL-6, IL-23 and IL-1β produced by primary tumour cells51,52. CXCR5+PD1+ICOS+CD40L+ T follicular helper (TFH) cells produce CXCL13 and IL-21, recruiting B cells into the tumour microenvironment (TME)56,57. TFH cells are essential for B cell activation, maturation and tertiary lymphoid structure (TLS) formation in tumours, and their presence is associated with improved outcomes29. T regulatory (Treg) cells have an immunosuppressive role in the TME. Treg cells can suppress effector cells through the release of IL-10, TGFβ and IL-35. High levels of CD25 on Treg cells compete with effector cells for local IL-2, so that effector cells might not have enough IL-2 to survive and function58–60. Treg cells also express CD39 and CD73, which convert extracellular ATP to adenosine and impair effector T cell function58,67. Co-expression of 4–1BB, GITR and neuropilin 1 (NRP1) on intratumoural Treg cells demonstrated an enhanced suppression function29,61,63. Immune-checkpoint receptors, such as TIM3 and CTLA4, are reportedly expressed by Treg cells in head and neck squamous cell carcinoma (HNSCC). Both are highly expressed by intratumoural Treg cells and able to suppress effector cell functions21,22,63,64. Therapeutically targeting Treg cells might help rejuvenate effector cell function. DC, dendritic cell; ICOS, inducible T cell co-stimulator; TCR, T cell receptor; TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domains.
