Fig. 5 |. The stromal microenvironment is functionally important for the HNSCC TME.
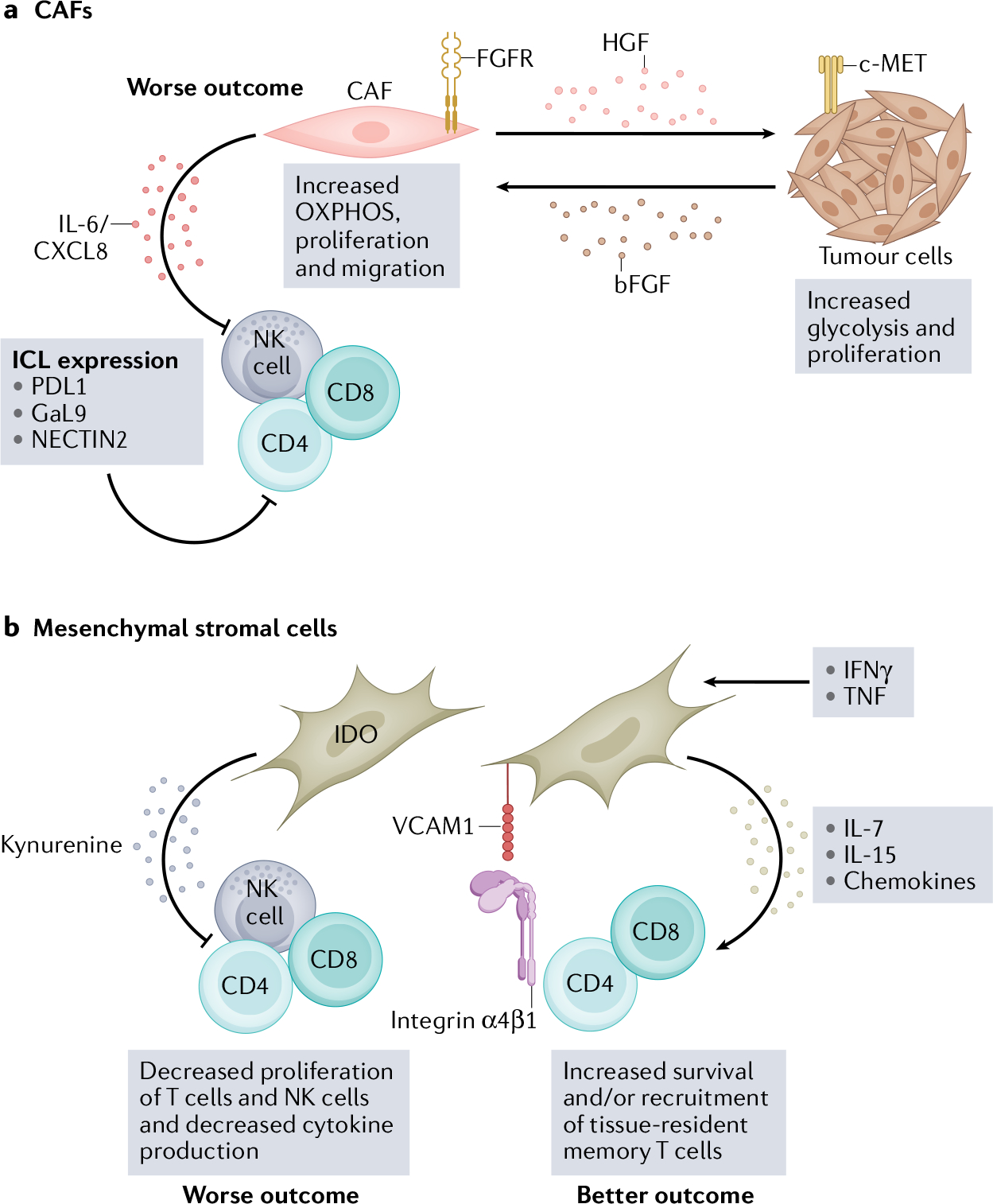
The head and neck squamous cell carcinoma (HNSCC) tumour microenvironment (TME) consists of a diverse stromal cell compartment — which includes cancer-associated fibroblasts (CAFs) and mesenchymal stromal cells (MSCs) — that interacts with neighbouring tumour cells and infiltrating immune cells106,143. These interactions primarily promote tumour growth, progression and metastases but, under some conditions, can provide a supportive environment for immune cells and recruit immune cells to the HNSCC TME132,141. Combining immune-checkpoint therapy with therapies that target the stromal compartment might improve patient outcomes in HNSCC. a, CAFs and tumour cells have a metabolic relationship mediated by hepatocyte growth factor (HGF) and basic fibroblast growth factor basic (bFGF), which increases oxidative phosphorylation (OXPHOS) in CAFs and glycolysis in tumour cells137. Increased oxidative phosphorylation in CAFs leads to IL-6 and CXCL8 production, which suppress immune cell function134,137. CAFs also express several immune-checkpoint ligands (ICL), including PDL1, galectin 9 (GaL9) and nectin cell adhesion molecule 2 (NECTIN2), which interact with corresponding inhibitory receptors on natural killer (NK) cells and T cells28. b, MSCs can also suppress immune cell function in vitro via indoleamine-pyrrole 2,3-dioxygenase (IDO), which metabolizes tryptophan into kynurenine and is cytotoxic to T cells and NK cells140,142,143. However, some in vitro studies suggest that MSCs can support tissue-resident memory T cells via IL-7 and IL-15 production in the presence of IFNγ and TNF144. VCAM1, vascular cell adhesion protein 1.
