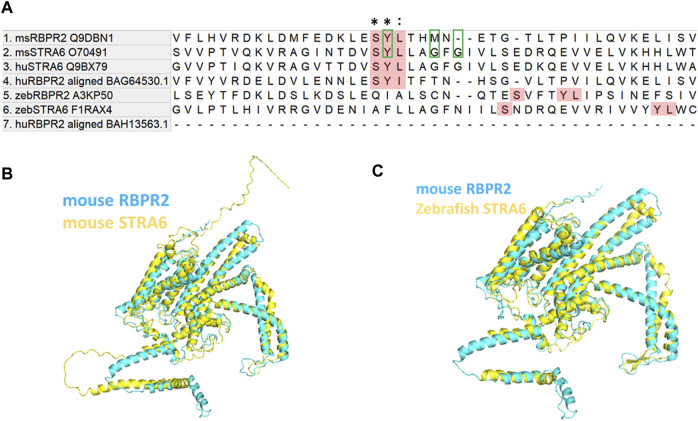
FIGURE 1.
Proposed RBP4 binding residues on RBPR2 are conserved across species. (A) Proposed RBP4 binding residues on RBPR2 (highlighted Red; amino acid residues S294, Y295 and L296) occurs in exon 11 of mouse RBPR2 (Alapatt et al., 2013). The multiple sequence alignment of mouse and human STRA6, RBPR2 sequences shows conserved residues (Tamura et al., 2021). * indicates conserved residues of the RBP4 binding motif; (colon) indicates strongly similar properties. The previously proposed RBP4 binding residues on mouse STRA6 (Tyrosine Y336, Glycine G340, and Glycine G342) are highlighted with green box. The zebrafish STRA6 and RBPR2 sequence were not highly conserved but surprisingly had an exact topological feature alignment with the mouse sequence to extracellular region of the receptor (https://www.uniprot.org/uniprotkb/Q9DBN1/entry#sequences). (B,C) Computer modeling and structure homology between RBPR2 (blue) and STRA6 (yellow) proteins.