Figure 4. Disruption of the two long-range synaptic complexes have opposite effects on DNA end-processing.
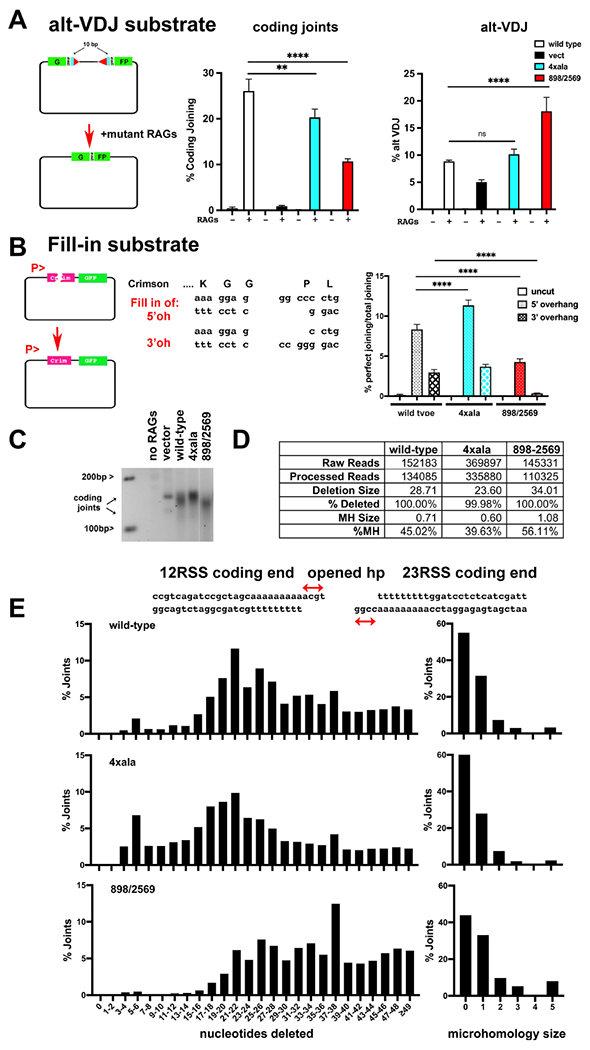
(A) Cartoon depicting the alt-VDJ substrate; restoration of the GFP reading frame requires nucleotide deletions of 10bp from either coding end and utilization of 9bp of short sequence homology. The fluorescent substrate 290-Crimson/ZS (coding joints) or alt-VDJ were utilized to detect coding end joining or alt-VDJ joining of hyper RAG mutant-induced DSBs in V3 cells expressing wild-type, 4Xala mutant, or 898/2659 mutant DNA-PKcs. With the alt-VDJ substrate, dsRED expression was co-transfected to control for transfection efficiency. Percent recombination represents %GFP/%RFP. (B) Cartoon depicting the Fill-in substrate that when cleaved with appropriate restriction enzymes will only restore Crimson expression if over-hanged ends are filled in and re-ligated to blunt end. Uncut or cleaved Fill-in substrate was transfected into cells expressing wild-type DNA-PKcs, the 4Xala, or 898/2569 mutants. Crimson and GFP expression was assessed by Flow cytometry 72 hours later and Crimson/GFP is expressed as %perfect joining. For (A+B) error bars indicate SEM from three independent experiments. **P<0.01; ****P<0.0001; ns=not significant in two-way ANOVA with Holm-Sidak correction. (C) 2.5% agarose electrophoresis of PCR amplification of coding joints from AT coding joint substrate from V3 stable transfectants expressing wild-type or mutant DNA-PKcs and then transfected with substrate and RAG1 and RAG2 expression constructs. Cells were harvested 72 hours after transfection; coding joint substrate was isolated by alkaline lysates, followed by PCR for coding joints. (D+E) Summary of amplicon sequencing of coding joints amplified from V3 transfectants expressing wild type, 4xAla, or 898/2569 DNA-PKcs. Results are averages of two separate experiments.
