Figure 5:
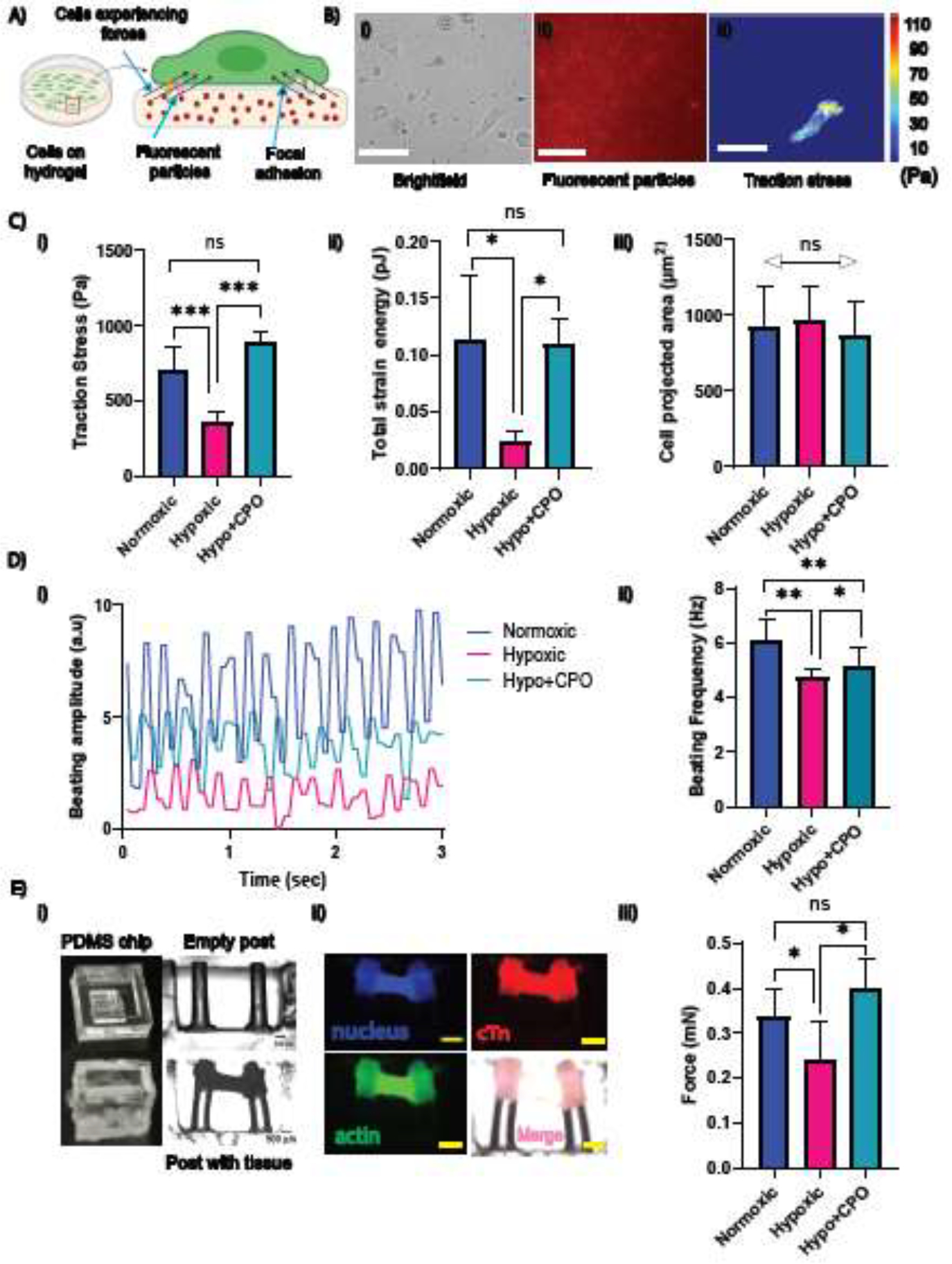
Restoring contractile mechanics of single CMs and cardiac tissue using CPO microparticles. (A) Schematic illustration depicting the cell traction force measurement of cells on the substrate. (B) Cells (left) cultured on a 10 kPa polyacrylamide-based 2D soft substrate with 200 nm fluorescent microparticles embedded within: (i) a brightfield image of cells, (ii) fluorescent microparticles, and (iii) a cell traction stress map obtained from the microparticles’ displacement field. Scale bar = 30 μm. (C) (i) traction force, (ii) contractile energy, and (iii) cell projected area of CMs with and without CPO microparticles in a hypoxic condition (n = 13 cells for each condition). (D) CMs created a monolayer for beating in (i) normoxia and hypoxia (ii) with and (iii) without microparticles showing higher amplitude with CPO particles. (iv) Average beating frequency in normoxic, hypoxic, and hypoxic with CPO microparticles (n = 8 movies for each). (E) Force generated by cardiac tissue measured by micro-post assay: (i) images captured for empty PDMS chip, micropillars without tissue, and brightfield image of cardiac tissue engineered between a pair of parallel micropillars. Displacement of the pillars due to tissue contraction is visible in the brightfield image of tissue construction. (Side view) (ii) Immunostaining images of cardiac tissue between micropillars are shown: actin (green), nucleus (blue), cTn (red), and merged all (pink). Scale bar = 500 μm (all images were captured with the same magnification). (iii) Forces in normoxia (p=0.055), hypoxia with and without CPO microparticles generated by the engineered tissue. The number of posts, n = 7, 4, and 4 normoxic, hypoxic and hypoxic with CPO microparticles, respectively. The student’s t-test was performed for significance. (* p ≤ 0.05, **, p ≤ 0.01; *** p < 0.001, ns p > 0.05.).
