Figure 4. In Vivo efficacy targeting CD133+ HT-29 colon cancer tumors with αCD133/αCD3 CSANs.
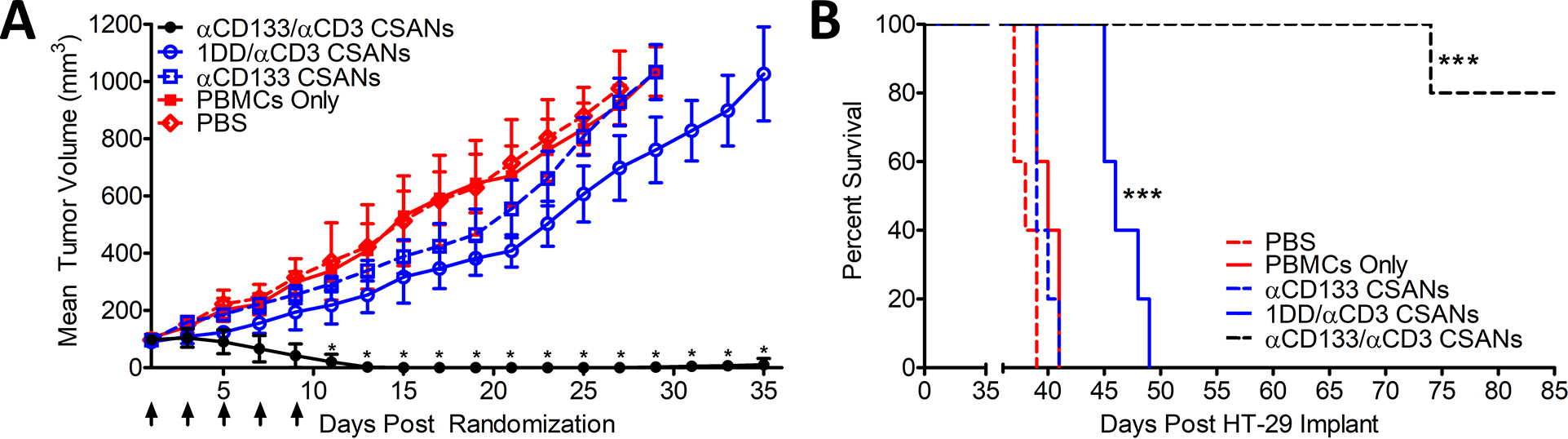
NSG mice were inoculated in the flank with 1.0×106 HT-29 cells. Cohorts were randomized when tumors were ~80 mm3 and IV inoculated with 20 million PBMCs. Treatments were initiated 4 days later, including: PBS, PBMC only (this experimental group acquired treatments of PBS only following the initial PBMC engraftment), 1 mg/kg αCD3 monospecific CSANs, 1 mg/kg αCD133 monospecific CSANs, and 1 mg/kg αEpCAM/αCD3 bispecific CSANs (n=5). Treatments were administered every 2 days for a total of 5 treatments. (A) Tumor growth was monitored every by caliper and recorded as mm3. *P<0.05 with respect to readings statistically significant from the 1DD/αCD3 CSAN control group, by 2-tailed Student’s t test. (B) Survival was monitored out to 85 days when graft vs host (GVH) disease symptoms were no longer manageable. Kaplan-Mier Survival Plot. *P<0.0001 with respect to PBS control, by log rank test. All in vivo experiments were performed independently and at least twice.
