Figure 2. The cryo-EM structure of WT SPOP shows three previously unidentified protein-protein interfaces.
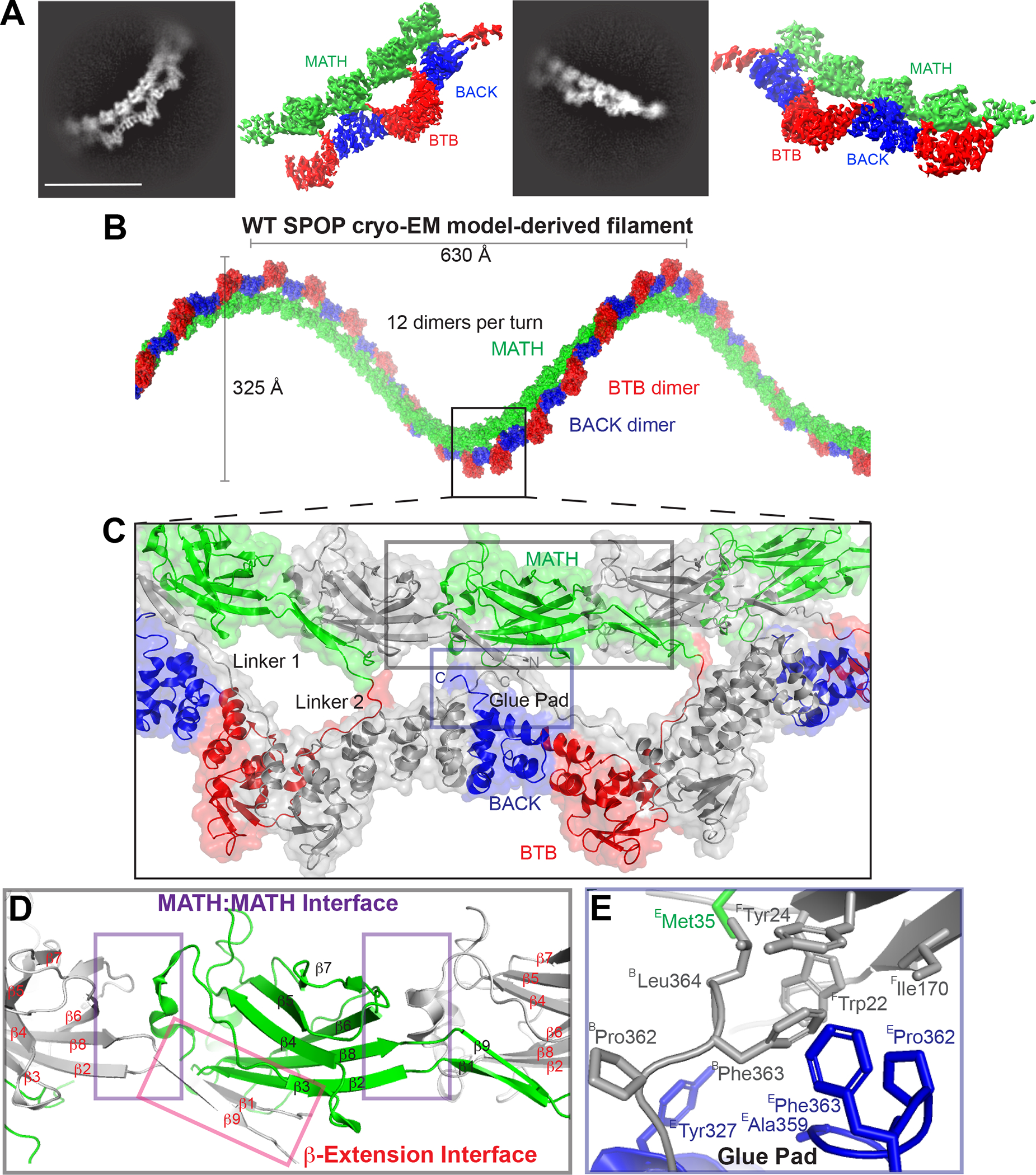
(A) Representative 2D classes and sharpened cryo-EM maps of WT-SPOP oligomers in similar orientation (see also Figure S1).
(B) The cryo-EM model of SPOP oligomers can be used to construct a left-handed helical filament by concatenation of tetramers.
(C) The structure reveals three previously unidentified protein interfaces that mediate extensive MATH:MATH interactions (grey inset box) and “glue” the MATH domains and the C-terminus into the repeating unit of the filament (blue inset box). The linker connecting the MATH domains with the oligomerization domains appears in two distinct repeating conformations; one interacts specifically with the BTB and BACK domains of an adjacent monomer (Linker 1), whereas the other interacts only with an adjacent BTB domain (Linker 2). Alternating monomers are colored grey for clarity.
(D) The MATH domains take on a continuous head-to-tail orientation along the filament with a repeating MATH:MATH interface (purple box). The β-extension interface (red box) mediates additional MATH:MATH self-association. Here, the N-terminus of SPOP, which was lacking from earlier studies, forms a well-ordered β-strand (β1) and a continuous β-sheet with the adjacent MATH domain as well as with what was in earlier crystal structures a disordered linker (now β9) following the structured MATH domain.
(E) Close-up view of the glue pad, which anchors the MATH domain into the repeating oligomerization domains through a hydrophobic interface formed by three polypeptide chains. (Superscripts on residue names indicate the protein chains.)
