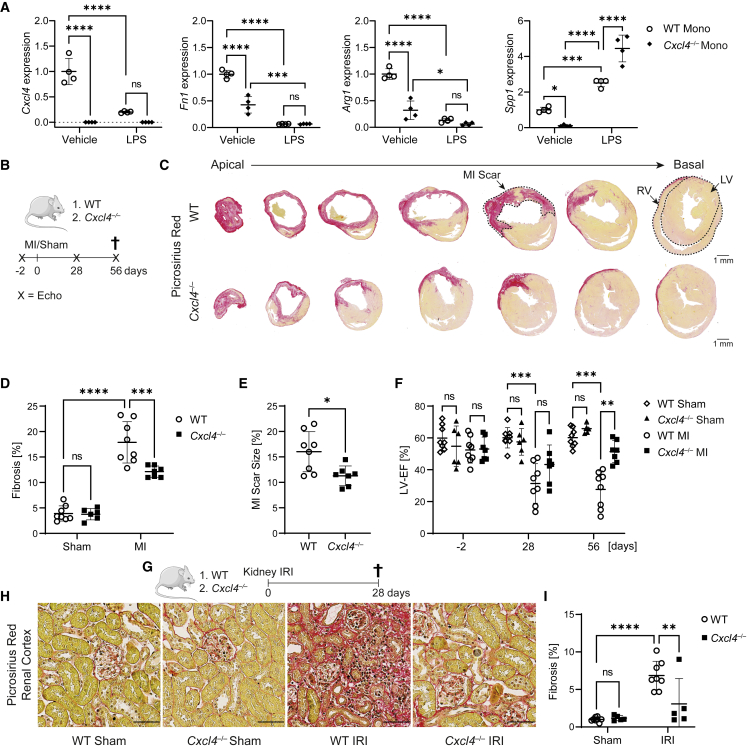
Figure 2.
Genetic loss of Cxcl4 mitigates organ fibrosis
(A) RT-qPCR analysis for Cxcl4, Fn1, Arg1, and Spp1 in CD11b+ monocytes isolated from WT or Cxcl4−/− PBMCs after stimulation with vehicle or LPS (n = 4). Mono, monocytes.
(B) Design of myocardial infarction experiments.
(C) Picrosirius red stained serial heart sections over seven levels from WT and Cxcl4−/− mice after MI. ECM is stained red. LV, left ventricle; RV, right ventricle. Scale bar = 1 mm.
(D) Fibrosis of serial heart sections in WT sham, Cxcl4−/− sham, WT MI, and Cxcl4−/− MI mice based on quantification of serial heart sections shown in (C). Quantification by spectral thresholding analysis of red ECM (WT Sham = 8; Cxcl4−/− Sham = 6; WT MI = 8; Cxcl4−/− MI = 7).
(E) MI scar sizes in WT and Cxcl4−/− mice based on quantification of serial heart sections shown in (C).
(F) Left ventricular ejection fraction (Simpsons) 2 days before, as well as 28 and 56 days after MI or sham surgery in WT and Cxcl4−/− mice.
(G) Experimental design of IRI experiments.
(H) Representative images of picrosirius red stained cortical kidney sections from WT and Cxcl4−/− mice after sham or IRI surgery. Scale bar = 50 μm.
(I) Kidney cortex fibrosis (in % of cortex area) after sham or IRI surgery by quantification of red ECM of scans shown in (H) (WT mice = 8, Cxcl4−/− mice = 5).
All quantitative data are shown as mean ± SD. For (A), (D), (F), and (I), two-way ANOVA was computed using Tukey corrections. For (E), a two-tailed unpaired t test was performed. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.