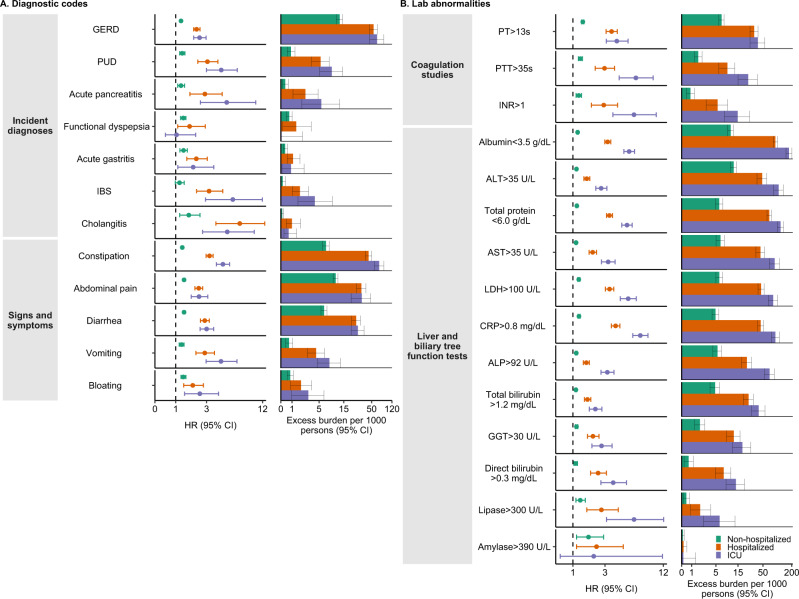
Fig. 4. Risks and 1-years burdens of incident post-acute COVID-19 gastrointestinal outcomes compared with the contemporary control cohort by care setting of the acute infection.
Risks and burdens were assessed at 1-year in mutually exclusive groups comprising non-hospitalized individuals with COVID-19 (green), individuals hospitalized for COVID-19 (orange) and individuals admitted to intensive care for COVID-19 during the acute phase (first 30 d) of COVID-19 (blue). Outcomes were ascertained 30 d after the COVID-19-positive test until the end of follow-up. The contemporary control cohort served as the referent category. Within the COVID-19 cohort, non-hospitalized (n = 131,915), hospitalized (n = 16,764), admitted to intensive care (n = 5389) and contemporary control cohort (n = 5,606,761). Panel A describes the risks and burdens of incident diagnoses and panel B describes the risks and burdens of incident laboratory abnormalities. Adjusted HRs (dots) and 95% (error bars) CIs are presented, as are estimated excess burdens (bars) and 95% CIs (error bars). Burdens are presented per 1000 persons at 12 months of follow up. The dashed line marks a HR of 1.00; lower limits of 95% CIs with values greater than 1.00 indicate significantly increased risk. GERD gastroesophageal reflux disorder, IBS irritable bowel syndrome, PT prothrombin time, PTT partial thromboplastin time, INR international normalized ratio, ALT alanine transaminase, AST aspartate transaminase, LDH lactate dehydrogenase, CRP c-reactive peptide, ALP alkaline phosphatase, GGT γ-glutamyl transferase.