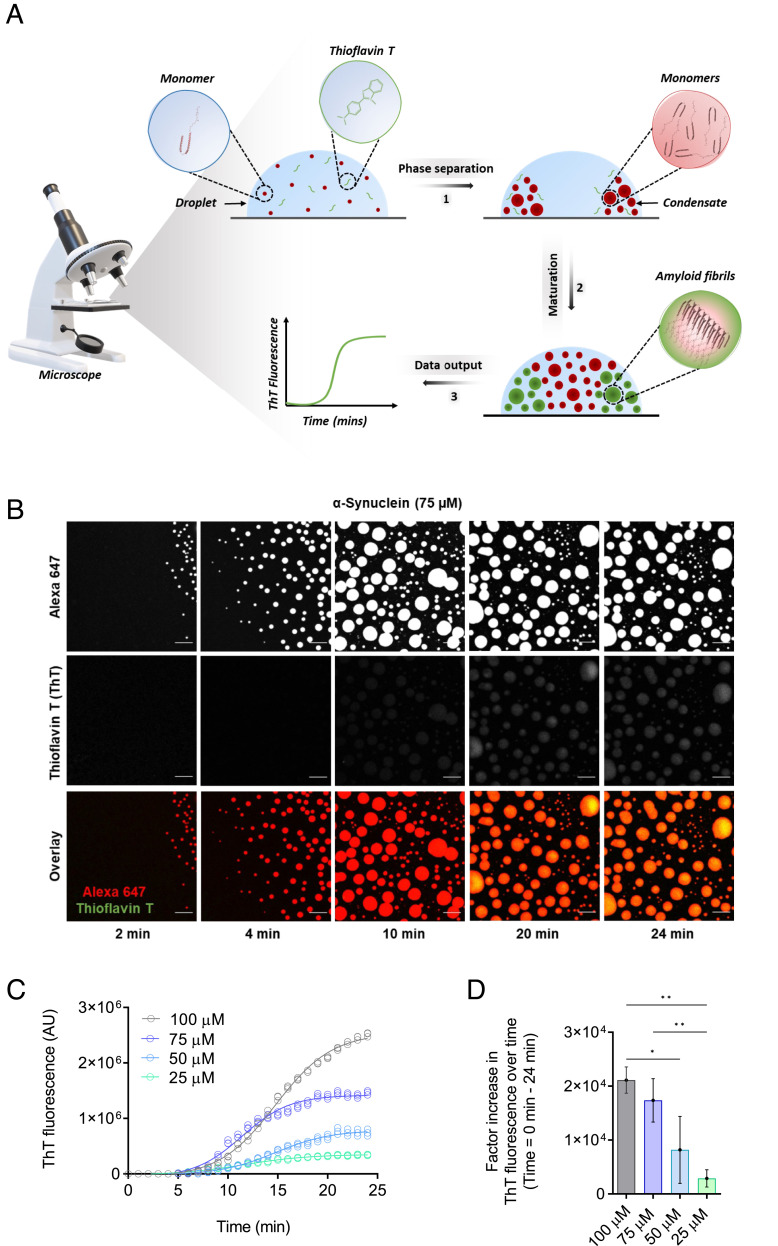
Fig. 1.
Development of a ThT-based assay to monitor α-synuclein aggregation within liquid condensates. (A) The assay has three components: 1) the phase separation of α-synuclein into dense liquid droplets (condensates) within dilute liquid droplets, which is monitored using Alexa Fluor 647 fluorescence, 2) the formation of α-synuclein amyloid fibrils over time within the condensates, and 3) the real-time assessment of amyloid formation is performed using ThT fluorescence. (B) Imaging of α-synuclein condensate formation (using Alexa Fluor 647 fluorescence) and aggregation (using ThT fluorescence) over time. In the presence of a crowding agent (10% PEG), α-synuclein monomers (75 μM) form a dense liquid phase surrounded by a dilute liquid phase. In the presence of 20 μM ThT, amyloid-containing condensates can be detected as an increase in the ThT fluorescence signal over time. The images represent an area of the sample that was tracked over time. (The scale bar represents 10 µm.) (C) Quantification of ThT fluorescence over time of the images shown in panel B for 75 μM (blue) α-synuclein. Subsequently, ThT emission for 100 (gray), 50 (cyan), and 25 (turquoise) μM α-synuclein were obtained in the same manner over 24 min. (D) Factor increase in ThT fluorescence intensities across the different concentrations tested (the ThT intensity at 24 min was subtracted by the ThT intensity at 0 min divided by the ThT intensity at 0 min). All experiments were performed in 50 mM Tris-HCl at pH 7.4 in the presence of 10% PEG and 20 μM ThT. The data represent the mean ± SEM of n = 3 individual experiments. One-way ANOVA., *P < 0.1, **P < 0.01.