Abstract
Coronavirus disease 19 (COVID-19), caused by the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2), has been implicated in having post-COVID-19 sequelae in both adults and children. There is a lack of good data on the prevalence and risk factors for post-COVID-19 sequelae in children. The authors aimed to review the current literature on post-COVID sequelae. The prevalence of post-COVID sequelae in children is highly variable among studies, with an average of 25%. The sequelae may affect many organ systems, though mood symptoms, fatigue, cough, dyspnea, and sleep problems are common. In many studies, it is difficult to establish a causal association due to the lack of a control group. Furthermore, it is difficult to differentiate whether the neuropsychiatric symptoms in children after COVID-19 are due to infection or a result of lockdowns and social restrictions imposed by the pandemic. Children with COVID-19 should be followed by a multidisciplinary team and screened for symptoms, followed by focused laboratory evaluations as needed. There is no specific treatment for the sequelae. Only symptomatic and supportive treatment is required in most cases. More research is necessary to standardize the definitions of sequelae, establish a causal association, assess various treatment options, and the effects of different virus variants, and finally, see the impact of vaccination on the sequelae.
Keywords: COVID-19, SARS-CoV-2, Long COVID, Post-COVID sequelae, MIS-C, Rehabilitation
Introduction
Persistent or new symptoms after acute coronavirus disease 19 (COVID-19) are a well-known entity in the adult population, with an estimated global prevalence of 0.43 (95% CI: 0.39, 0.46) [1]. In literature, this entity is known by different names: post-acute COVID-19 sequelae, post-COVID syndrome, post-COVID condition, long COVID, COVID long-haulers, post-acute sequelae of SARS-CoV-2 infection, etc. Post-COVID sequelae (PCS) are now increasingly recognized in children, though the data are still limited [2]. Contrary to common belief, it has been reported in children with mild or asymptomatic infections [3, 4]. Therefore, irrespective of initial severity, PCS in children could be a significant problem. It can involve any organ system and have a broad clinical presentation. It can lead to a poor quality of life and burden the existing health care system.
There is an urgent need to develop comprehensive evidence on the long-term outcome of COVID-19, including risk factors, pathophysiology, its impact on children's health, and possible treatment. It would be helpful for the clinician, researcher, and policymaker to draw further management and preventive strategies. Therefore, this narrative review was conducted to provide an update on current evidence on PCS in children.
Definition
The definition of PCS is still evolving. Most studies defined PCS as the persistence of acute symptoms or the development of new symptoms beyond 4 wk after an acute COVID-19 infection [5]. Recently, the National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), and Royal College of General Practitioners (RCGP) released a collaborative guideline that defines COVID-19 symptoms as follows:
Acute symptoms: ≤ 4 wk
Ongoing symptoms (subacute): 4–12 wk
Post-COVID syndrome (chronic) symptoms: > 12 wk
In this review, PCS includes ongoing and post-COVID syndrome (i.e., symptoms > 4 wk).
Epidemiology
Prevalence
The prevalence of PCS varies widely in children, ranging from 0.4%–100% in different studies. Several factors contribute to this variation; the basis of initial COVID-19 diagnosis (RT-PCR, antigen, or serology), clinic presentation (asymptomatic vs. symptomatic), the severity of disease (mild, moderate, or severe), treatment required (outpatient, hospitalized and/or intensive care), the inclusion of control group, and study methodology [5–7]. The initial evidence was primarily based on case series, cross-sectional questionnaires, or retrospective studies [5, 8–11]; however, over time, prospective cohort studies and reports from the national database have contributed to it [4, 12, 13].
A large prospective cohort study from Russia (n = 518) used the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) Global pediatric COVID-19 follow-up survey and documented persistent symptoms in 24.3% of children (< 18 y) after a median follow-up of 256 d [7]. A recent multicentric prospective cohort study (n = 1884) conducted at 36 pediatric emergency departments (ED) in 8 countries included children (< 18 y) after 90 d of ED visits and found that 5.8 % of children had persistent symptoms. These symptoms were statistically significant in SARS-CoV-2 test-positive children (9.8%) than in test-negative children (4.6%) [2].
A study from Denmark (LongCOVIDKidsDK) noted that test-positive adolescents (n = 24 315) had a 1.22 (95% CI 1.15–1.30) times increased odds of having one symptom for ≥ 2 mo than matched test-negative adolescents (n = 97 257) [14]. A recent study based on multi-institutional electronic health records included 659286 children < 21 y, of which 59893 (9.1%) were RT-PCR–positive and 599393 (90.9%) RT-PCR–negative from 28 to 179 d after infection. The prevalence of at least one symptom was 41.9% (95% CI 41.4–42.4) among children who tested positive and 38.2% (95% CI 38.1–38.4) among those who tested negative [8].
One meta-analysis, including 17 studies (n = 23,141) with a median follow-up of 125 d, documented that pooled prevalence ranged from 15%–47% [9]. Another meta-analysis included 14 studies (n = 19246) in children, with a prevalence ranging from 4%–66% [10]. A recent systematic review, including 21 studies with 80,071 children and adolescents, reported the prevalence of long COVID as 25.2% (95% CI 18.2 % to 33.0%) [12].
Risk Factors
The risk factors for PCS in children are different from those in adults. A retrospective study observed obesity, anxiety, and baseline dyspnea as significant risk factors for PCS [6]. In a few studies, the female gender was significantly associated with PCS in children [6, 15]. However, in a large study from the USA (n = 781,419), the female gender did not appear to be a risk factor for PCS [16]. In a prospective study, older age (> 6 y) [OR 2.49, 95% CI 1.02 to 6.72] and allergic disorder [OR 1.67, 95% CI 1.04 to 2.67] were seen as significant risk factors for persistent symptoms [7]. In a multicentric study, age > 14 y [aOR, 2.67; 95% CI, 1.43–4.99], having ≥ 4 initial symptoms [aOR, 2.35; 95% CI, 1.28–4.31] and hospitalization for ≥ 48 h [aOR, 2.67; 95% CI, 1.63–4.38] were observed risk factors for PCS [2]. In a recent study, the highest strength of PCS was associated with age < 5 y, intensive care unit (ICU) admission, and underlying complex chronic conditions [8].
Pathophysiology of PCS
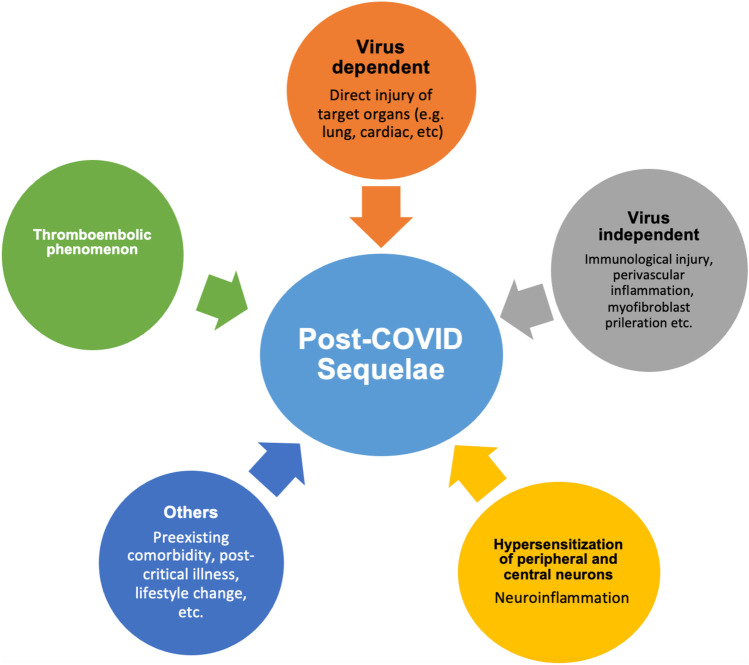
The pathophysiology of PCS in children is not yet precisely known. The postulated mechanisms are depicted in Fig. 1. It includes the persistence of the initial pathological mechanism, virus-dependent tissue injury, and virus-independent immunological injury (cytokine storm). Perivascular inflammation and thromboembolic phenomenon may have a role in the pathogenesis of PCS. Aberrant myofibroblast proliferation may be responsible for fibrotic lung injury. Another possible explanation is the hypersensitization of peripheral and central neurons resulting from SARS-CoV–induced neuroinflammation. It was reflected in a study in patients with COVID-19–related neurological complications, which showed altered cortical signal and neuroinflammation on MRI and hypometabolism in PET scans in respective brain areas [13]. Other factors like pre-existing comorbidity, lifestyle change, social isolation, postintensive care admission, nutrition, decreased physical activity, medication overuse, and increased media use may also contribute to PCS.
Fig. 1.
Postulated pathophysiology mechanisms of post-COVID sequelae
Clinical Presentations
COVID-19 sequelae impact a child's health directly or indirectly and can have a spectrum of clinical manifestations [7]. It can be broadly divided into two groups; respiratory and nonrespiratory system involvement [2, 14]. In most children, more than one organ system is involved [2, 4, 6, 7, 16]. Organ-wise manifestations of PCS are summarized in Table 1.
Table 1.
Summary of organ-wise manifestation of PCS
| Organ systems | Prevalence Range (%) |
Common symptoms |
|---|---|---|
| General symptoms [2, 7–9, 11, 15] | 0.4–84 | Fatigue, loss of appetite, weight loss, poor quality of life, fever, weakness |
| Respiratory [4, 5, 9, 10, 12, 14, 16, 20] | 1–97 | Cough, dyspnea, chest pain, wheezing, shortness of breath, exertional dyspnea, sore throat, chest tightness |
| Cardiovascular [2, 6, 7, 11, 16] | 1–11.2 | Variation in heart rate, chest pain, exertional dyspnea, palpitation |
| Neurological [5, 8, 11–15] | 3–16.2 | Headache, dizziness, difficulty in concentrating, seizure, stroke, muscular weakness, vision problem, change in taste and smell, acute demyelinating encephalomyelitis, GBS |
| Hematological [7, 11] |
0.6 OR 1.18 |
Bleeding, thromboembolic, increased coagulation |
| Gastrointestinal [4, 6, 8, 10, 12] | 5.5–13.8 | Constipation, diarrhea, nausea, vomiting and abdominal pain, dysphagia |
| Dermatological [4, 7, 8, 10] | 3.6–15 | Skin rash, hair loss |
| Musculoskeletal [2, 4, 7–9, 11] | 0.2–10 | Joint and persistent muscular pain |
| Sleep [4, 5, 7, 10] | 2–16 | Insomnia, hypersomnia |
| Psychosocial [2] | 0.4–10 | Anxiety and depression |
| Renal [11] | aOR 1.32 | Acute or unspecified renal failure |
| Endocrine [11, 17] | HR 1.31–2.66 | Diabetes type 1 and 2 |
| MIS-C [13, 20, 21] | 20–25 | Cardiac (coronary artery dilation), |
| 38 | Neurological (headache, altered sensorium, seizure, stroke, muscle weakness, hypo- or hyperreflexia, abnormal posturing and) | |
| 29–31 | Dysphagia |
aOR Adjusted odd ratio, GBS Guillain–Barré syndrome, HR Hazard ratio, MIS-C Multisystem inflammatory syndrome in children
Respiratory Sequelae
The most common respiratory symptoms are cough, chest pain, difficulty breathing, exertional dyspnea, chest tightness, wheezing, nasal discharge, and sore throat. Fibrotic lung damage is commonly reported in adults but rarely reported in children.
In a large prospective study, respiratory sequelae were described in 2.3% of the children, including a persistent cough, difficulty breathing, and chest pain [7]. In another study, persistent and/or exertional dyspnea was the most common symptom observed in all children (97%), followed by cough (52%) and exercise intolerance (48%) [16]. A study from Australia found cough (50%) and chest pain (25%) as the predominant PCS symptoms [3]. Chest tightness (1%) and nasal discharge (1%) were common respiratory sequelae in a study from Switzerland [5]. In a meta-analysis, the cough was present in 17% (95% CI 7–27) and dyspnea in 43% (95% CI 18%–68%) children. In another meta-analysis, cough, chest tightness, and runny nose range from 1%–30%, 1%–31%, and 1%–2%, respectively [10]. In a recent multicentric study, respiratory sequelae were observed in 2% of children, which includes cough (0.7%), difficulty in breathing (0.7%), wheezing or asthma (0.4%), and chest pain (0.3%) [2]. A cohort study using electronic data at 40 centers observed a prevalence ratio (PR) of 1.72 [99% CI, 1.17–2.51] for shortness of breath in < 20 y old (n = 338 024) after 31–150 d of COVID-19 compared to no infection [17].
Cardiovascular System (CVS) Sequelae
Prospective research in children has documented CVS sequelae in 1.9%, which included variation in heart rate (2%) and palpitation (1.5%) [7]. A study based on cardiac MRI has shown evidence of myocarditis in 60% of the COVID-19 survivor. However, these findings may be confounded by vaccine-induced myocarditis. In a study, children who tested positive were more strongly associated with an increased risk of myocarditis (aHR, 3.10; 95% CI, 1.94–4.96) than tested negative [8]. In a recent study, children with COVID-19 infection had a 2.01, 1.99, 1.87, and 1.16 times increased risk of acute pulmonary embolism, myocarditis and cardiomyopathy, venous thromboembolic event, and dysrhythmias, respectively compared to children without COVID-19 [11].
General Sequelae
The common sequelae are fatigue or tiredness, fever, weakness, poor appetite, poor quality of life, and weight loss. Though it varies in children, fatigue was the most common sequelae in many studies, ranging from 0.7%–84.4% [2, 5, 7, 14, 15]. In a study, children with a history of COVID-19 infection had 1.05 times increased risk of fatigue and malaise than children without COVID-19 during follow-up [11]. In a meta-analysis, fatigue and loss of weight or appetite were found in 3%–87% and 2%–50% of children [10]. In a recent study, children with tested positive were at increased risk of fatigue (aHR, 1.24; 95% CI, 1.13–1.35) and fever (aHR, 1.22; 95% CI, 1.16–1.28) [8].
Neurological Sequelae
It is common and most debilitating in children. In a study from Italy, the headache was present in 10% of children, followed by concentration difficulties (10%) and changes in smell and taste (4%) [4]. A meta-analysis described headache, cognitive problems, and loss of smell in 35%, 26%, and 18% of children, respectively [9]. In another meta-analysis, headache, concentration difficulties, and disturbing smells were found in 3%–80%, 2%–81%, and 3%–26% of children [11]. In a study, children with a history of COVID-19 infection had 1.17 times the risk of loss of taste and smell compared to children without COVID-19 during follow-up [11]. Other reported manifestations are seizures, Guillain–Barré syndrome (GBS), demyelinating syndrome, and autoimmune encephalitis.
Sleep Disturbance
It is one of the commonly reported symptoms in children. In one study, sleep disturbance was observed in 2% of the children [5]. Another study observed sleep problems in 7.2% of the children, including insomnia in 6.9% and hypersomnia in 3.2% [17]. A meta-analysis found sleep disturbance in 2%–63% of children [10].
Gastrointestinal (GI) Sequelae
The frequently reported symptoms are diarrhea, nausea, and pain abdomen [4, 5, 7]. A study showed stomach pain in 1% of the children [5]. In a prospective study, GI sequelae were documented in 4.4% of the children, which consisted of constipation, diarrhea, pain abdomen, nausea, and vomiting [7]. Dysphagia has been observed as sequelae in children who require mechanical ventilation or who develop multisystem inflammatory syndrome in children (MIS-C). In a meta-analysis, abdominal pain and diarrhea were found in 25% and 15% of children, respectively [9]. In a recent study, children who tested positive were at increased risk of diarrhea (aHR, 1.18; 95% CI, 1.09–1.29) [8].
Renal Sequelae
It is rarely reported in children but can occur. In a study from the USA, acute and unspecified renal failure was 1.32 (aOR) more common in children with COVID-19 infection than those without COVID-19 during follow-up [11].
Hematological Sequelae
Bleeding is the most commonly reported symptom. A prospective study documented bleeding in 0.6% of the children. Menstrual irregularity in adolescent girls was also reported [7]. In a study, children with a history of COVID-19 infection had 1.18 times increased coagulation and hemorrhagic phenomenon compared to children without COVID-19 during follow-up [11].
Dermatological Sequlae
Skin rash and hair loss are frequently reported as dermatological sequelae. In a prospective study, skin involvement was noted in 3.6% of the children, including hyperhidrosis (3.39%), skin rash (2.6%), hair loss (2.4%), bilateral conjunctivitis (0.4%), and a lump on the toe (0.2%) [7]. A meta-analysis found skin rash in 2%–52% of children [12]. In a recent study, children with tested positive were at increased risk of skin rash (aHR, 1.26; 95% CI, 1.15–1.38) and hair loss (aHR, 1.58; 95% CI, 1.24–2.01) than those tested negative [8].
Musculoskeletal Sequelae
Joint pain and muscular pain are the most commonly seen sequelae symptoms. A study has documented musculoskeletal sequelae in 1.8% of children, including joint pain or swelling (2%) and persistent muscle pain (1.2%) [7]. In a study, children with a history of COVID-19 infection had 1.02 times increased risk of musculoskeletal pain than children without COVID-19 during follow-up [14]. In another study, muscle pain was seen in 10% while joint pain in 7% of the children [4]. A multicentric study observed muscle and joint pain in 0.2% of the children [2]. In a meta-analysis, myalgia was found in 25% of children [9]. In another meta-analysis, myalgia or arthralgia was found in 1%–61% of children [10]. In a recent study, children who tested positive were at increased risk of myositis (aHR, 2.59; 95% CI, 1.37–4.89) than tested negative [8].
Psychological Sequelae
In a multicentric study, anxiety or depression was observed in 0.4% of the children [2]. In a descriptive review, the authors highlighted that there was an increased prevalence of depression (94%), conduct disorder (92%), anxiety (87%), and post-traumatic stress disorder (66%) post-COVID.
Endocrine Sequelae
In a study, children with COVID-19 infection have a 1.23 and 1.17 risk of developing type-1 and 2 diabetes, respectively, compared to children without COVID-19 during follow-up [11]. Another study, based on two medical claims databases (IQVIA and HealthVerity), observed that children (> 18 y) with > 30 d following acute infection had an increased risk of newly diagnosed diabetes with a hazard ratio (HR) of 2.66 (95% CI = 1.98–3.56) in IQVIA and HR of 1.31 (95% CI = 1.20–1.44) in HealthVerity databases compared to children without COVID-19 [18]. In a cohort study, the risk of type-2 diabetes was 2.14 (99% CI, 1.13–4.06) in < 20 y old compared to a person with no infection [17].
A recent systematic review, including 21 studies with 80,071 children and adolescents, reported common post-COVID symptoms as mood symptoms, fatigue, and sleep problems in 16.5%, 9.7%, and 8.4%, respectively [12].
Sequelae of Multisystem Inflammatory Syndrome in Children (MIS-C)
MIS-C is itself a serious complication of COVID-19 [19]. CVS is the most common sequelae of MIS-C. Like Kawasaki disease (KD), coronary artery aneurysms and dilation have been documented in 20%–25% of children [20]. Neurological sequelae, including headache, altered sensorium, seizure, stroke, and muscle weakness, have been observed in 15%–20% [21]. Otolaryngological sequelae have been seen in MIS-C. A recent review reported dysphagia in 29%–31% of children with MIS-C. A single-center study of 50 children with MIS-C documented dysphonia in 16% and dysphagia in 8%. In a separate study (n = 50), persistent dysphonia, dysphagia, and altered smell were present in 30% of the children with MIS-C [22].
A retrospective study from the UK followed 46 children (< 18 y) with MIS-C for 6 mo. Normal systemic inflammation (97.8%), normal echocardiography finding (96%), GI symptoms (13%), neurological sequelae (38%), low 6-min walk test (45%), and severe emotional sequelae (18%) were observed at the end of the study. No children had renal, hematological, or otolaryngology symptoms by 6 mo. In neurological sequelae, proximal myopathy (17.3%), hyperreflexia (19.5%), abnormal eye movement (15.2%), difficulty in walking (8.7%), abnormal posturing (6.5%), hyporeflexia (4.3%), sensory abnormality (4.3%), facial muscle (2.1%) and upper limb weakness (2.1%) were documented at 6-mo of follow-up [23].
Assessment and Monitoring of PCS
PCS involves many organs, so a multidisciplinary team is needed at COVID-19 follow-up clinics. It is suggested that every child with COVID-19 should be seen in multidisciplinary follow-up clinics at least 4 wk and 12 wk after the infection. Children who had sequelae should be followed up later on also. The evaluation in follow-up clinics should be “symptom-directed.” The clinics should include a questionnaire and physical examination screening to identify the affected organ/s. The questionnaire may include symptoms, duration of symptoms, any factors that exacerbate the symptoms, absence from school, absence from work by parents due to the child’s illness, hospitalization, impairment of daily activities, and any pre-existing disease. Pulmonary function tests (spirometry, impulse oscillometry, etc.) are usually recommended in all children with PCS, and other assessments are tailored as per the system involved [24]. These may include a hemogram, renal and liver function tests, inflammatory markers (C-reactive protein, ferritin, ESR, etc.), creatine kinase, electrocardiography, echocardiography, sleep studies, neuropsychiatric evaluation, ultrasonography of the abdomen and lung, chest radiography, CT of the chest, and dermatological assessment. A few studies have included a 6-min walk test as a routine test to evaluate PCS [6, 16]. Some children may require further evaluation with cardiothoracic exercise testing and diffusion studies.
Assessment is followed by frequent (every 3–6 mo) monitoring of any abnormality detected.
Management of PCS
There is hardly any study that has evaluated the treatment for COVID-19 sequelae. The usual management is symptomatic and supportive, according to symptomatology. If a child develops wheezing after a COVID-19 infection, inhaled corticosteroids and bronchodilators may be used. Children may need rehabilitation services for some neurological and respiratory sequelae.
Persistent olfactory dysfunction may be dealt with by olfactory training that consists of short, repetitive, and consistent exposure to various smells.
General management of postural tachycardia syndrome includes increasing intravascular volume (more fluid intake, high salt diet), blood volume redistribution (positioning of the body, compression stockings), avoiding triggers (like large meals), and regular physical activity. In refractory cases, drugs such as fludrocortisone and beta-blockers may be used.
Postexertional malaise and fatigue may need relaxation techniques and an activity and symptom diary to assess stress limits. The therapy for post-COVID myalgias, arthralgias, and myositis is not well established.
Prevention of PCS
There are limited data on the prevention of PCS. As discussed above, most risk factors are nonmodifiable, except for pre-infection disease. So, at present, a possible preventive measure is to treat the pre-existing condition appropriately to keep the disease in check. The role of the vaccine in preventing PCS is not yet known.
Future Directions in Understanding and Managing PCS
COVID-19 is a new disease, and it is difficult to predict the course of its sequelae. Most studies have shown a steady decline in the prevalence of persistent symptoms over time [2–6, 18]; however, the maximum reported follow-up duration was 12 mo only. Therefore, it is currently unknown how long these symptoms will persist and what the long-term outcome of COVID-19 in children will be. Furthermore, in many post-COVID symptoms, it is difficult to establish causality, as long-lasting symptoms have also been reported in controls. The long-lasting effects of lockdown and social isolation with decreased exposure to common allergens and microbes are still unknown. Studies evaluating the long-lasting symptoms of COVID-19 compared to other viral infections are lacking. The role of COVID-19 vaccination in preventing the sequelae is yet to be determined. Studies are required to understand why only a few children develop sequelae. There is a need for long-term follow-up data on children with COVID-19, with and without any sequelae.
Authors’ Contributions
KRJ conceptualized the study, literature review, data synthesis, prepared initial manuscript; PK did the literature review and data synthesis, and prepared initial manuscript. Both authors critically reviewed the manuscript and approved it for publication. KRJ will act as the guarantor for this paper.
Declarations
Conflict of Interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. J Infect Dis. 2022;226:1593–1607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funk AL, Kuppermann N, Florin TA, et al. Pediatric Emergency Research Network–COVID-19 Study Team. Post-COVID-19 conditions among children 90 days after SARS-CoV-2 infection. JAMA Netw Open. 2022;5:e2223253. [DOI] [PMC free article] [PubMed]
- 3.Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. 2021;5:e22–3. doi: 10.1016/S2352-4642(21)00124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110:2208–11. doi: 10.1111/apa.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA. 2021;326:869–71. doi: 10.1001/jama.2021.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palacios S, Krivchenia K, Eisner M, et al. Long-term pulmonary sequelae in adolescents post-SARS-CoV-2 infection. Pediatr Pulmonol. 2022;57:2455–63. doi: 10.1002/ppul.26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osmanov IM, Spiridonova E, Bobkova P, et al; Sechenov StopCOVID Research Team. Risk factors for the post-COVID-19 condition in previously hospitalized children using the ISARIC Global follow-up protocol: a prospective cohort study. Eur Respir J. 2022;59:2101341. [DOI] [PMC free article] [PubMed]
- 8.Rao S, Lee GM, Razzaghi H, et al. Clinical features and burden of postacute sequelae of SARS-CoV-2 infection in children and adolescents. JAMA Pediatr. 2022;176:1000–9. doi: 10.1001/jamapediatrics.2022.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behnood SA, Shafran R, Bennett SD, et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: A meta-analysis of controlled and uncontrolled studies. J Infect. 2022;84:158–70. doi: 10.1016/j.jinf.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. 2021;40:e482–7. doi: 10.1097/INF.0000000000003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kompaniyets L, Bull-Otterson L, Boehmer TK, et al. Post-COVID-19 symptoms and conditions among children and adolescents - united states, march 1, 2020-january 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:993–9. doi: 10.15585/mmwr.mm7131a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Leon S, Wegman-Ostrosky T, Ayuzo Del Valle NC, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12:9950. doi: 10.1038/s41598-022-13495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song WJ, Hui CKM, Hull JH, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med. 2021;9:533–44. doi: 10.1016/S2213-2600(21)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenson T, Shafran R, De Stavola B, et al. CLoCk Consortium members. Long COVID and the mental and physical health of children and young people: National matched cohort study protocol (the CLoCk study). BMJ Open. 2021;11:e052838. [DOI] [PMC free article] [PubMed]
- 15.Ludvigsson JF. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2021;110:914–21. doi: 10.1111/apa.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeftinDobkin SC, Collaco JM, McGrath-Morrow SA. Protracted respiratory findings in children post-SARS-CoV-2 infection. Pediatr Pulmonol. 2021;56:3682–7. doi: 10.1002/ppul.25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez-Romieu AC, Carton TW, Saydah S, et al. Prevalence of select new symptoms and conditions among persons aged younger than 20 years and 20 years or older at 31 to 150 days after testing positive or negative for SARS-CoV-2. JAMA Netw Open. 2022;5:e2147053. doi: 10.1001/jamanetworkopen.2021.47053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5:708–18. doi: 10.1016/S2352-4642(21)00198-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagri NK, Khan M, Pandey RM, Lodha R, Kabra SK, MIS-C study group. Initial immunomodulation and outcome of children with multisystem inflammatory syndrome related to COVID-19: A multisite study from India. Indian J Pediatr. 2022;89:1236–42. [DOI] [PMC free article] [PubMed]
- 20.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology Clinical Guidance for multisystem inflammatory syndrome in children associated with SARS–CoV-2 and hyperinflammation in pediatric COVID-19: Version 1. Arthritis Rheumatol. 2020;72:1791–805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin JE, Asfour A, Sewell TB, et al. Neurological issues in children with COVID-19. Neurosci Lett. 2021;743:135567. doi: 10.1016/j.neulet.2020.135567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheong RCT, Jephson C, Frauenfelder C, et al. Otolaryngologic manifestations in pediatric inflammatory multisystem syndrome temporally associated with COVID-19. JAMA Otolaryngol Head Neck Surg. 2021;147:482–4. doi: 10.1001/jamaoto.2020.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penner J, Abdel-Mannan O, Grant K, et al. GOSH PIMS-TS MDT Group. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health. 2021;5:473–82. [DOI] [PubMed]
- 24.Fainardi V, Meoli A, Chiopris G, et al. Long COVID in children and adolescents. Life (Basel) 2022;12:285. doi: 10.3390/life12020285. [DOI] [PMC free article] [PubMed] [Google Scholar]