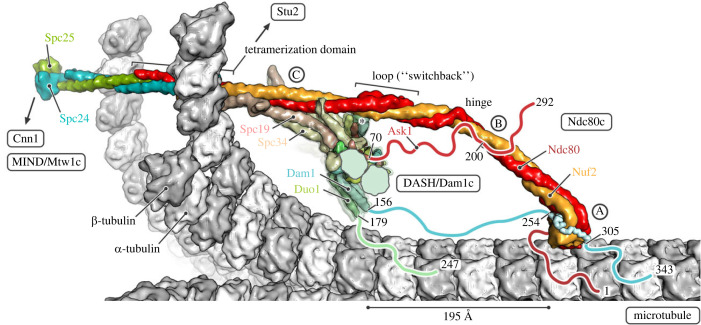
Figure 5.
Model of the interactions of yeast Ndc80c with DASH/Dam1c and a kinetochore microtubule (MT). In the close-up view, a single Ndc80c in ‘end-on’ attachment to a curled MT is shown. α- and β-tubulin subunits are coloured white and grey, respectively. Ndc80c subunits are coloured as in figure 1. The DASH/Dam1c ring, composed of 17 heterodecameric complexes, is partially cut for illustration. Flexible extensions for which interactions with their binding partner are not yet structurally characterized were omitted from the models and drawn schematically as lines, including the N terminus of Ndc80 (red) and the C termini of Ask1 (red), Dam1 (cyan) and Duo1 (green). The N termini of Dam1 and Duo1 are not shown; an asterisk indicates their location next to the Ndc80c loop. Relevant residues are numbered. The three interactions between Ndc80c and DASH/Dam1c are labelled A, B and C [1,16]. Interaction A is defined by binding of the C-terminal Dam1 extension to the Ndc80 : Nuf2 head domains. The segment observed in the crystal structure here is shown in ribbon representation. Interaction B is between the C terminus of Ask1 and the Ndc80 : Nuf2 coiled-coil between the head and the hinge. Interaction C involves binding of the Spc19 : Spc34 protrusion domain to the Ndc80 : Nuf2 coiled-coil between the loop and tetramerization domains. See Material and Methods for details of how the model was obtained.