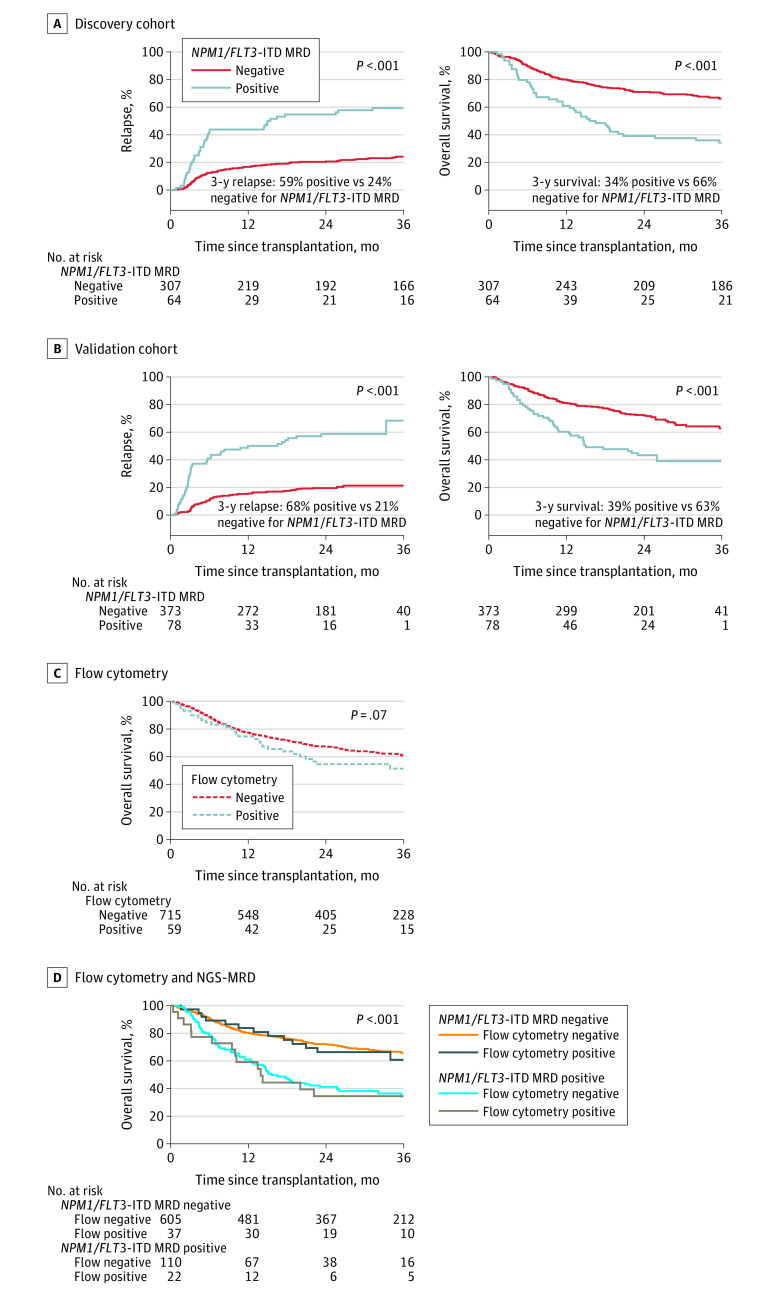
Figure 2. Impact of NPM1 and FLT3 Internal Tandem Duplication Next-Generation Sequencing–Measurable Residual Disease Status on Clinical Outcomes.
Patients with NPM1 mutated and/or FLT3 internal tandem duplication (NPM1/FLT3-ITD) acute myeloid leukemia (n = 822) with measurable residual disease (MRD) defined by persistent variants detectable by next-generation sequencing (NGS) in remission blood prior to transplant (NPM1/FLT3-ITD MRD positive) had significantly higher rates of relapse and decreased overall survival after transplant than patients with no variants detected (NPM1/FLT3-ITD MRD negative).
A, The median time of observation was 61 months (IQR, 48-64 months) for patients who tested positive for MRD and 60 months (IQR, 48-72 months) for patients who tested negative for MRD in the discovery cohort.
B, The median time of observation was 24 months (IQR, 24-26 months) for patients who tested positive for MRD and 25 months (IQR, 24-28 months) for patients who tested negative for MRD in the validation cohort.
C and D, Results of flow cytometry performed in remission prior to transplant do not stratify for overall survival in this cohort (panel C) and do not add additional prognostic value to the results of DNA sequencing (panel D). The median time of observation was 26 months (IQR, 24-47 months) for patients who tested positive for MRD by flow cytometry and 35 months (IQR, 24-57 months) for patients who tested negative. The median time of observation was 36 months (IQR, 24-59 months) for patients who tested negative by NGS-MRD and flow cytometry, 26 months (IQR, 24-46 months) for patients who tested positive by NGS-MRD and flow cytometry, 37 months (IQR, 24-61 months) for those who tested positive by NGS-MRD and flow cytometry, and 25 months (IQR, 24-45 months) for patients who tested negative.