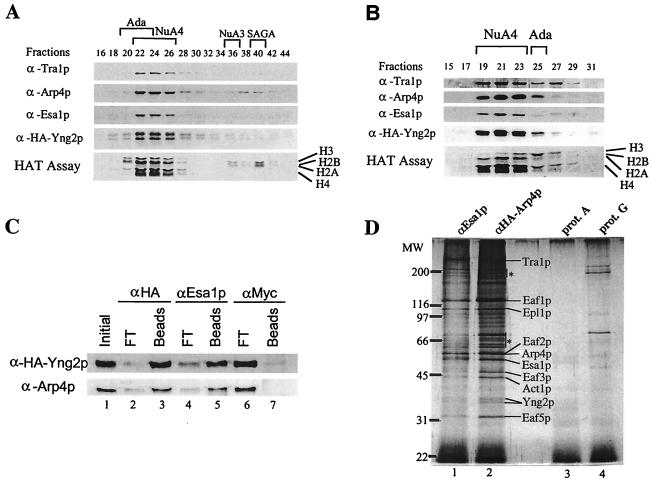
FIG. 2.
Yng2p is a stable stoichiometric subunit of the NuA4 acetyltransferase complex. (A) Extract from BY4741 strain, transformed with HA-Yng2p-expressing plasmid (pAN102), was fractionated over nickel-agarose, followed by MonoQ column fractionation. MonoQ fractions were tested for HAT activity and by Western blotting with the indicated antisera. (B) MonoQ NuA4 peak fractions were pooled and loaded on a Superose-6 gel filtration column. HAT assays and Western blotting were performed as in panel A. (C) Coimmunoprecipitation of HA-Yng2p and Arp4p. Equal amounts of the Superose-6 fraction 21 described in panel B were incubated with anti-HA, anti-Esa1p, and anti-Myc. After washes, equivalent amounts of Initial, Beads (bound), and FT (flowthrough; unbound) were analyzed by Western blotting with anti-HA and anti-Arp4p. (D) Yng2p was also determined to be present in affinity-purified NuA4 complex by using HA-Arp4p as antigen. The Superose-6 peak fraction of NuA4 obtained from an HA-Arp4p-expressing strain (DY3558 [see reference 15 for details]) was immunoprecipitated with anti-Esa1p–protein A-Sepharose or with anti-HA–protein G-Sepharose. Silver staining of immunopurified complexes is shown. Specific NuA4 bands are indicated. Nonspecific bands present in the controls are indicated by asterisks.