Figure 6. KIF5C(1–560)-Δ6 induces more microtubule damage than KIF5C(1–560)-WT.
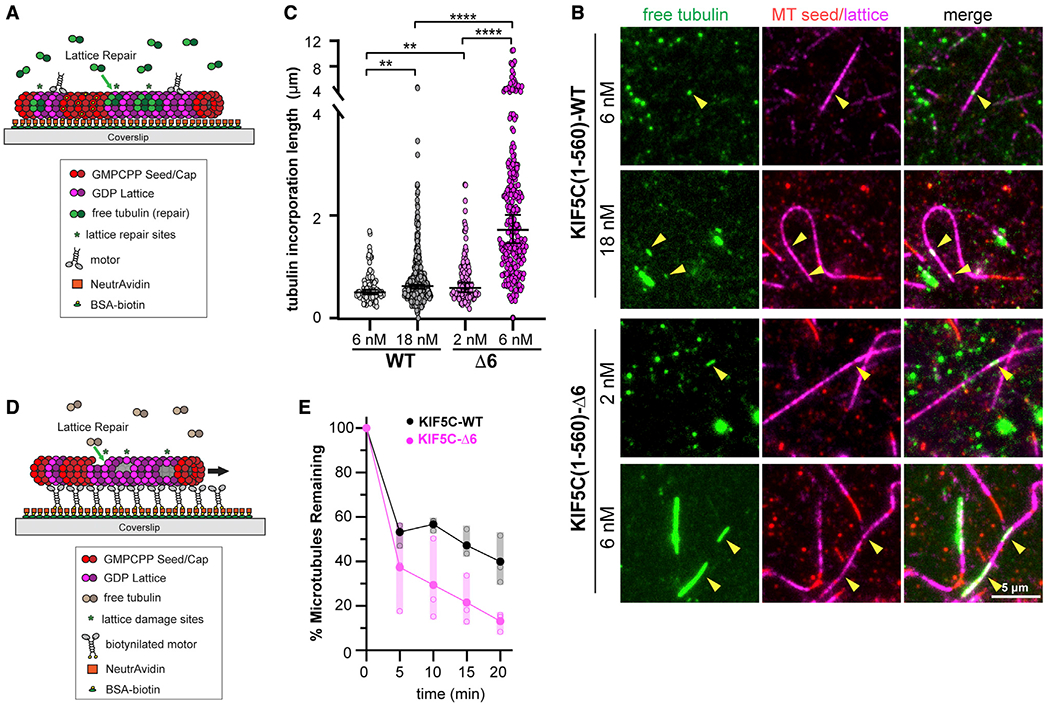
(A–C) Microtubule repair assay.
(A) Schematic of assay. GDP-tubulin microtubules (GMPCPP seeds and GMPCPP caps) were attached to a coverslip. Purified Halo-FLAG-tagged KIF5C(1–560)-WT (6 or 18 nM) or KIF5C(1–560)-Δ6 (2 or 6 nM) motors were added to the flow chamber in the presence of 10 μM 488 nm labeled soluble tubulin. Static images were obtained after 7 min of free tubulin incorporation into motor-driven damage sites.
(B) Representative images. Green, microtubule repair sites; red, GMPCPP seeds; magenta, GDP-tubulin lattice. Arrowheads indicate microtubule repair sites. Scale bars, 5 μm.
(C) Quantification of the length of microtubule repair sites. WT, n = 375; Δ6, n = 246 repair sites across three independent experiments. **p < 0.01 and ****p < 0.0001 (two-tailed t test).
(D and E) Microtubule destruction assay.
(D) Schematic of assay. Biotinylated KIF5C(1–560)-WT or KIF5C(1–560)-Δ6 motors attached to a coverslip drive the gliding of GDP-tubulin microtubules (GMPCPP seeds and GMPCPP caps) in the presence of 7 μM soluble unlabeled tubulin.
(E) Quantification of motor-driven microtubule destruction over time. The total length of microtubules per field of view was measured, and the percentage of microtubules remaining in the chamber at the indicated time points was calculated and plotted as a dot plot. Black, KIF5C(1–560)-WT; magenta, KIF5C(1–560)-Δ6. Solid dots indicate the average loss of microtubule length across three independent trials, and open dots indicate loss of microtubule length for an individual trial. See also Figure S7A.
