Abstract
BACKGROUND:
Noninvasive ventilation (NIV) is the reference standard treatment for most situations of chronic respiratory failure. NIV settings must be titrated to both preserve upper-airway patency and control hypoventilation. Automatic adjustment of pressure support (PS) and expiratory positive airway pressure (EPAP) may facilitate the initiation and follow-up of domiciliary NIV. However, whether the automatic-adjustment algorithms embedded into current devices accurately detect, respond to, and score common sleep-related respiratory events remains unclear.
METHODS:
A bench was set up to simulate central hypopnea (CH), central apnea (CA), obstructive hypopnea (OH), and obstructive apnea (OA). Four home ventilators were evaluated, with their dedicated modes for automatic PS and EPAP adjustment.
RESULTS:
All 4 devices increased PS during CH, CA, and OH. However, PS adjustment varied widely in magnitude, with tidal volumes within 100 ± 20% of the target being provided by only 3 devices for CH, one for CA, and one for OH. Two devices increased EPAP for OH and 3 for OA, including one that also increased EPAP for CA. Only 2 devices scored residual hypopnea after simulated CA, and only one scored a residual event after OH. One device scored no event.
CONCLUSIONS:
Current NIV devices differed markedly in their responses to, and reporting of, standardized sleep-related respiratory events. Further improvements in embedded NIV algorithms are needed to allow more widespread out-of-laboratory initiation and follow-up of NIV.
Keywords: automated algorithms, bench study, chronic respiratory failure, noninvasive ventilation, sleep-related respiratory events
Introduction
Noninvasive ventilation (NIV) has been shown to improve outcomes of patients with most types of chronic respiratory failure1-6 and is currently the standard of care for chronic alveolar hypoventilation.7-12 Sleep-related respiratory events such as central and/or obstructive apnea and hypopnea affect the efficiency of NIV.13 NIV settings should, therefore, be individualized to both control nocturnal hypoventilation and prevent or treat such respiratory events.14,15
Polysomnography (PSG) is the recommended method for identifying optimal NIV pressure settings.16,17 However, patients face long waiting lists for PSG, which is also costly.18 In addition, the inspiratory and expiratory pressures must strike a compromise between minimizing pressure-related adverse effects on the one hand and preventing upper-airway obstruction and/or treating central events during sleep on the other. Needs may change within a given night and from night to night depending on body position, sleep stage, nasal patency, inspiratory muscle efficiency, and other factors, such as the ingestion of alcohol or hypnotic agents that may be used at home.15
To replace PSG titration in sleep laboratories, simpler tools allowing remote monitoring of home NIV parameters and residual respiratory events, as well as adjustments of settings, would considerably facilitate the initiation and follow-up of long-term home NIV.19-22 Manufacturers have developed sophisticated algorithms embedded within NIV devices. These algorithms can automatically adjust basic settings such as expiratory positive airway pressure (EPAP) and pressure support (PS) in response to changes in upper-airway mechanics and air flow.15,18 However, these algorithms vary widely across manufacturers, who do not always provide detailed descriptions of them or their updates.23 For example, some devices adapt their parameters cycle by cycle after identifying an event, whereas others seek to avoid events throughout the NIV session by continuously adjusting the settings even when no events occur. These embedded algorithms will improve care only if they provide good-quality monitoring and setting adjustment.18,24,25 More data on this point are needed.
We, therefore, designed a bench study to qualitatively evaluate the appropriateness of automatic setting adjustments by several NIV devices in response to common sleep-related respiratory events. We also assessed the accuracy of device detection and scoring of these events.
QUICK LOOK.
Current Knowledge
Automatic adjustment of pressure support and expiratory positive airway pressure may facilitate the initiation and follow-up of home noninvasive ventilation (NIV). Devices have a wide array of algorithms for detection of sleep-related events and responses which may have varied effectiveness.
What This Paper Contributes to Our Knowledge
Current NIV devices varied substantially in their responses and reports of standardized sleep-related respiratory events. Further improvements in embedded NIV algorithms are needed to allow broader out-of-laboratory initiation and follow-up of home NIV.
Methods
Bench Model
We used a 2-chamber Michigan test lung (MII Vent Aid TTL; Michigan Instruments, Grand Rapids, Michigan). The driving chamber was connected to, and ventilated by, a dedicated ventilator (Elisée 150, ResMed, Bella Vista, New South Wales, Australia), and the experimental chamber was connected to the tested NIV device (Fig. 1).
Fig. 1.
Experimental setup. aw = respiratory flow; aw airway pressure.
Both chambers were physically connected to each other by a small metal component that allowed the driving chamber to lift the experimental chamber, thereby simulating the spontaneous inspiratory effort, as described previously.25 The generation of positive pressure in the driving chamber decreased the pressure in the experimental chamber, triggering a pressure supported breath. The driving ventilator was in pressure controlled mode with the following settings: inspiratory pressure 10 cm H2O, PEEP 5 cm H2O (total inspiratory pressure 15 cm H2O), breathing frequency 16 breaths/min, and inspiratory time 1.2 s (inspiration:expiration 1:2).
Between the experimental chamber and the tested NIV device, the following were connected in sequence: a parabolic resistor (5 cm H2O/L/s) (PneuFlo Rp5, Michigan Instruments), a flow sensor, a collapsible chamber acting as a Starling resistor to allow on-demand partial circuit obstruction,26,27 a shut-off valve allowing on-demand complete circuit closure, and a pressure sensor (Fig. 1). Compliance of the experimental chamber was set at 60 mL/cm H2O. The tested NIV devices were connected to the system through a 15-mm circuit and a standard 4-mm diameter intentional leak port. Additional information is provided in the supplementary material (see related supplementary materials at http://www.rcjournal.com).
Devices and Ventilatory Modes
We evaluated 4 ventilators: Vivo 45 (v. 3.1.4–3.1.4, Breas Medical, Mölnlycke, Sweden), Prisma VENT40 (v. 3.7.00.14, Löwenstein Medical Technology, Hamburg, Germany), BiPAP A40 Pro (v. 1.1.3, Philips Respironics, Murrysville, Pennsylvania,), and Stellar 150 (v. SX483-0252, ResMed). All devices were set with their dedicated modes for automatic adjustment of PS based on a prespecified target tidal volume (VT) and of EPAP based on upper-airway patency. Automatic calibration of the devices and circuit was performed according to manufacturer recommendations before study data acquisition.
Standardization of Settings Across Devices
The purpose of this study was to assess the response of each device to standardized respiratory events rather than the accuracy of delivered VT. When using ventilatory modes with a set target VT, PS is adjusted based on VT recorded by the device, as opposed to actual VT. However, in several studies, the accuracy of VT monitoring differed across devices.28-32 To overcome this potential source of bias, we standardized the target VT settings as follows.
Before each experiment, a baseline run was performed with the target VT and PS set to the minimum values available on the devices (300, 100, 200, and 160 mL; and 2, 0, 2, and 0 cm H2O, for the Vivo 45, Prisma VENT40, BiPAP A40 Pro, and Stellar150, respectively). During this run, ventilation of the experimental chamber was, therefore, mainly related to the spontaneous respiratory effort generated by the driving ventilator. The VT value recorded by the tested device during this run was defined as the baseline VT. For all experimental conditions, the target VT was then set at 90% of the baseline VT, rounded to the nearest 10.
During the baseline runs, VT recorded by the device was 360, 270, 320, and 290 mL for the Vivo 45, Prisma VENT40, BiPAP A40 Pro, and Stellar 150, respectively. Accordingly, during the experiments, target VT was 320, 240, 290, and 260 mL for these devices, respectively. Device-recorded VT values were used only for this standardization procedure: all VT data recorded for the experiments were derived from respiratory flow ( aw) measured by the pneumotachograph.
For all experimental conditions and for each device, the minimum and maximum PS values were 2 and 14 cm H2O, respectively; and the minimum and maximum EPAP values were 4 and 14 cm H2O, respectively. Table 1 reports the device settings.
Table 1.
Settings for Each of the 4 Tested Devices
Experimental Conditions
For each device, after a 3-min stabilization period with stable ventilation, 4 respiratory events were simulated in the following order: central hypopnea (CH), central apnea (CA), obstructive hypopnea (OH), and obstructive apnea (OA). Each event lasted 5 breaths (about 15−20 s), and events were separated by 1 min of simulated spontaneous ventilation.
CH was simulated by halving the PS of the driving ventilator and CA by switching the driving ventilator to CPAP mode. For OH, increasing the pressure about the collapsible chamber allowed precise control of its degree of opening with variation of the Starling resistance, thereby simulating upper-airway collapse independently from EPAP, to achieve a 50–60% VT decrease from the pre-event period of stable ventilation to the first respiratory cycle of the OH event. OA was simulated by closing the shut-off valve located between the intentional leak port and the experimental chamber.
Finally, we performed additional experiments to evaluate whether the duration of the simulated events affected devices responses. We performed CA and OA experiments in which each event lasted 1 min instead of 15–20 s. For these experiments, the tested devices were switched on and off between the events to ensure that the starting PS and EPAP were similar for CA and OA.
Data Acquisition
aw was measured close to the experimental chamber using a pneumotachograph (Fleish no. 2; Fleish, Lausanne, Switzerland) connected to a differential pressure transducer (Validyne DP45 ± 2.25 cm H2O; Validyne, Northridge, California). Airway pressure ( aw) was measured using another pressure transducer (Validyne DP45 ± 56 cm H2O) positioned between the shut-off valve and the circuit. The sensors were calibrated according to the manufacturers’ recommendations before the experiments. The signals were digitized at 200 Hz using an analog/digital system (MP100, Biopac Systems, Goleta, California) and recorded on a microcomputer for further analysis. The raw data from the devices were downloaded and analyzed via the manufacturers’ dedicated software to identify whether the simulated events were detected and scored.
Data Analysis
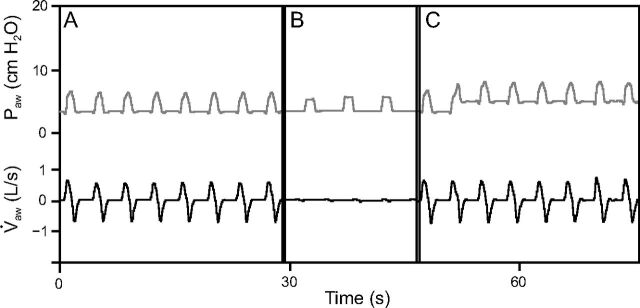
For each simulated event, 3 periods of interest were defined: 30 s before the event (pre-event period), 15–20 s during the event (event period), and 30 s after the event (post-event period) (Fig. 2). All respiratory cycles during these 3 periods were included in the analysis. For each respiratory cycle, we determined inspiratory positive airway pressure (IPAP) defined as the maximum aw during the inspiratory plateau, EPAP as the mean aw during the last 500 ms of expiration, PS as IPAP(n) – EPAP(n-1), VT as the integral of flow over time, total cycle time (Ttot) as the time between 2 insufflations from the tested device, and breathing frequency as 60/Ttot. VT overshoot was defined as a > 20% difference from the mean pre-event VT.
Fig. 2.
Data acquisition and periods of interest. Example of data recorded during a simulated episode of obstructive apnea. The periods of interest were the 30 s preceding the event (A: pre-event period), 15–20 s of event duration (B: event period), and 30 s following the event (C: post-event period). aw = respiratory flow; aw = airway pressure.
Detection of an event by the device was defined as the occurrence of an automatic setting adjustment between the pre-event and post-event periods and/or a scored residual event in the software report. According to current recommendations,16,33 an appropriate device response to an event was defined as the following changes from the pre-event to post-event periods: for CH, a PS increase; for CA, a PS increase combined with backup breathing frequency activation; for OH, an EPAP increase combined, when the actual VT was below target, with a PS increase; and for OA, an EPAP increase. When necessary, the manufacturers were contacted to obtain additional information on the device algorithms that might help us understand our findings.
Statistical Analysis
The data are described as mean ± SD. Most of the results presented are for descriptive purposes only. For instance, the description of the VT decrease induced by the simulation of an event (relative to mean pre-event VT), or the VT reached during the simulation of an event (relative to target VT), did not necessitate using statistical analyses. However, to highlight the adjustment of settings that occurred between the pre-event and post-event periods, comparisons of variable values during these 2 periods were performed with the paired-sampled t test. Analyses were performed using Jamovi (version 1.6.15) and R (version 4.0, R Foundation for Statistical Computing, Vienna, Austria). Two-sided P values < .05 were considered statistically significant.
Results
Consistency of Experimental Conditions Across Devices
Before simulation of the first event, that is, during the first pre-event period for CH, VT was above target for all 4 devices (Table 2). Despite this, none of the 4 devices delivered the set minimum PS. All 4 devices provided similar PS levels: 2.5 ± 0, 2.6 ± 0.1, 2.7 ± 0, and 2.8 ± 0.1 cm H2O for the Vivo 45, Prisma VENT40, BiPAP A40 Pro, and Stellar 150, respectively, (Table 2). The mean first pre-event VT calculated from the pneumotachograph data were 360 mL (± 5%) for all tested devices (Table 2).
Table 2.
Automated Adjustment of Settings and Tidal Volume Variations From the Pre-Event to the Post-Event Period for Each of the 4 Tested Devices
Compared with the mean pre-event VT, VT during the first cycle of the simulated CH was lower by 39, 38, 42, and 40% for the Vivo 45, Prisma VENT40, BiPAP A40 Pro, and Stellar 150, respectively. Corresponding decreases were 65, 70, 70, and 67%, respectively, for CA; 53, 51, 48, and 48%, respectively, for OH; and 100% with all devices for OA.
Device Responses to the Simulated Events
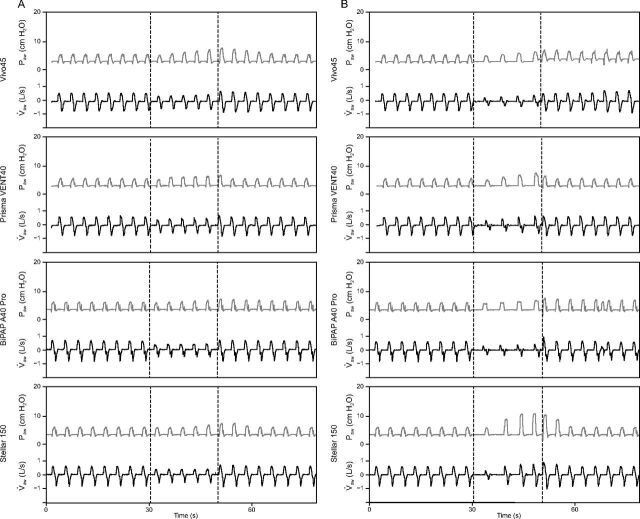
Table 2 reports the responses of each device to the simulated events, and Figures 3 and 4 show the pressure and flow variations recorded for central and obstructive events, respectively. Figure 5 diagrams the dynamic behavior of the devices from the first to the last cycle of each period of interest.
Fig. 3.
Device responses to central hypopnea and apnea. 3A: Central hypopnea. 3B: Central apnea. The dashed vertical lines show the beginning and end of the event period. aw = respiratory flow; aw = airway pressure.
Fig. 4.
Device responses to obstructive hypopnea and apnea. 4A: Obstructive hypopnea. 4B: Obstructive apnea. The dashed vertical lines show the beginning and end of the event period. aw = respiratory flow; aw = airway pressure.
Fig. 5.
Diagram of dynamic device behavior from the first to the last cycle of each period of interest. The tidal volume (VT) values presented are derived from the pneumotachograph and presented as a percentage of the target VT (set on the tested NIV device) or of the mean pre-event VT (measured by the pneumotachograph). A: Vivo45, B: Prisma VENT40, and C: BiPAP A40 Pro. *Note that the greater expiratory positive airway pressure level at the beginning of the obstructive hypopnea and obstructive apnea simulations was not induced by the events but was related to the algorithm of the device as described in supplementary Figure 1. D: Stellar 150. *VT = 294% of target VT; 226% of mean pre-event VT; PS = pressure support; EPAP = expiratory positive airway pressure.
All 4 devices increased PS during CH, CA, and OH. However, the magnitude of the PS increase varied considerably across devices (Table 2 and Fig. 5). For CH, all devices except the BiPAP A40 Pro reached 100 ± 20% of the target VT at the last cycle of the event (Fig. 5). VT overshoot occurred during the first post-event cycle with the Vivo 45 (Fig. 5A). For CA, only the Prisma VENT40 reached 100 ± 20% of the target VT at the last cycle of the event. The Vivo 45 and BiPAP A40 Pro did not increase PS sufficiently to reach the target VT during the event (Figs. 5A through 5C). Conversely, the PS increase by the Stellar 150 in response to CA resulted in a VT of 148% of the target at the last event cycle and in a VT overshoot at the first post-event cycle (Fig. 5D). For OH, only the Prisma VENT40 increased PS sufficiently to reach 100 ± 20% of the target VT during the event (Fig. 5). Both the Prisma VENT40 and Stellar 150 induced VT overshoot after the event (Figs. 5B and 5D). For OA, the BiPAP A40 Pro and Prisma VENT40 did not significantly modify PS (Table 2), whereas Vivo 45 and Stellar 150 increased PS during the event, with the latter inducing VT overshoot at the first post-event cycle (Fig. 5D).
All devices activated the backup breathing frequency for CA and OA (Figs. 5A through 5D). The Stellar 150 increased breathing frequency during CA and OA, achieving the target breathing frequency (Fig. 5D). The Vivo 45 also activated backup breathing frequency for OH, inducing asynchronies during the event (Fig. 4A).
No device adjusted EPAP for central events, with the exception of the Vivo 45, which increased EPAP in response to CA (Table 2 and Fig. 5). Only the Vivo 45 and the Stellar 150 increased EPAP in response to OH (Table 2). All devices except the BiPAP A40 Pro increased EPAP in response to OA. Consistent with its algorithm, BiPAP A40 Pro adjusted EPAP independently of the occurrence of any simulated event (Supplementary Fig. 1, see related supplementary materials at http://www.rcjournal.com).
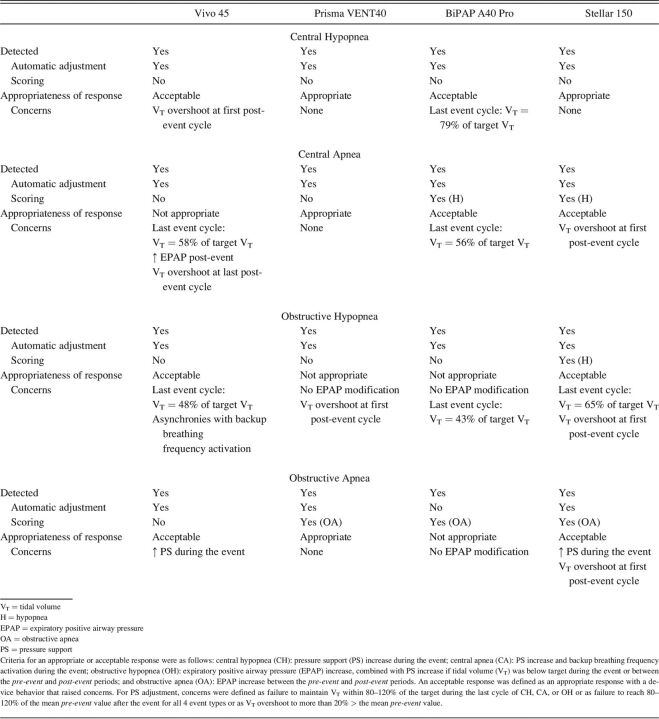
Detection and Scoring of the Simulated Events
Table 3 reports event detection and the appropriateness of device responses. Central events induced PS increases with all 4 devices and were, therefore, considered detected. For CH, none of the tested devices scored residual events. Although the mean VT drop during CA compared with the mean pre-event VT was 56, 54, 62, and 16% for the Vivo 45, Prisma VENT40, BiPAP A40 Pro, and Stellar 150, respectively, (Fig. 3B) only the BiPAP A40 Pro and Stellar 150 scored residual hypopnea in their software reports (Table 3).
Table 3.
Event Detection and Appropriateness of Device Responses to Simulated Respiratory Events
OH induced automatic setting adjustments by all 4 devices and were, therefore, considered detected. The mean VT drop during OH was 62, 46, 56, and 48% for Vivo 45, Prisma VENT40, BiPAP A40 Pro, and Stellar 150, respectively. Only the Stellar 150 scored residual hypopnea in its report (Table 3).
For OA, all devices were considered to have detected the event based on the occurrence of setting adjustments (Vivo 45, Prisma VENT40, Stellar 150) and/or on appropriate scoring in the device software (Prisma VENT40, BiPAP A40 Pro, Stellar 150).
Effects of Event Duration and Pre-Event Pressure
During the 1-min CA, all 4 devices increased PS, providing 95, 127, 100, and 103% of the target VT at the end of the event for the Vivo 45, Prisma VENT40, BiPAP A40 Pro, and Stellar150, respectively. The target VT was reached after 18, 5, 11, and 2 cycles for these 4 devices, respectively. VT overshoot at the first post-event cycle occurred with all 4 devices (Supplementary Fig. 2A through 2D, see related supplementary materials at http://www.rcjournal.com). During the 1-min OA, the BiPAP A40 Pro and Prisma VENT40 did not significantly modify PS, whereas the Vivo 45 and Stellar 150 increased PS over the course of the event, with the latter overshooting the VT target at the first post-event cycle (Supplementary Fig. 2D).
All 4 devices activated backup breathing frequency for the 1-min CA and OA (Supplementary Fig. 2A through 2D). The Stellar 150 increased breathing frequency during both events, achieving the target breathing frequency (Supplementary Fig. 2D).
Again, the only device that adjusted EPAP for central events was Vivo 45, which increased EPAP in response to CA (Supplementary Fig. 2A). The mean EPAP before the 1-min OA was 3.3 ± 0, 3.4 ± 0, 3.4 ± 0, and 3.4 ± 0 cm H2O for the Vivo 45, Prisma VENT40, BiPAP A40 Pro, and Stellar150, respectively. All devices except the BiPAP A40 Pro increased EPAP in response to OA. The EPAP increase from the mean pre-event period to the last post-event cycle was 1.7, 1.2, 0, and 5.4 cm H2O for the Vivo 45, Prisma VENT40, BiPAP A40 Pro, and Stellar150, respectively, (compared to 0.9, 1.4, 0, and 2.8 cm H2O for the 15–20 s OA simulation, respectively).
Despite the longer duration of the events and the comparable pre-event EPAP values across devices and between CA and OA, the pattern of EPAP adjustment during the 1-min events was similar to that of the 15–20 s events (Supplementary Figs. 2A through 2D). The scoring of these events by the device software was also the same as for the 15–20 s events.
Discussion
This bench study demonstrated that automatic responses to simulated sleep-related respiratory events varied considerably across 4 NIV devices. Moreover, variability also occurred in the device software reports of events. For all 4 devices, the responses to the simulated events raise concerns about the appropriateness of automatic adjustments in clinical practice. This finding is somewhat surprising given the existence of clear recommendations about setting adjustments in response to sleep-related apneas and hypopneas due to central or obstructive mechanisms.16,33
We evaluated whether the automatic responses of the devices to events responsible for a VT decrease, namely CH, CA, and OH, were appropriate. To correct a VT decrease, PS must be increased. All devices increased PS in these situations, and as such, their responses were appropriate. Although quantitative assessment of the accuracy of delivered VT, which has already been evaluated in dedicated bench studies,28-32 was beyond the scope of the present work, it is worth noting that differences between the delivered VT and the target VT were marked and common. VT fell below the set target in some cases and, in others, was maintained only at the cost of an overshoot after event termination. These VT variations may adversely affect patients, for instance, by altering sleep architecture. Achieving the complex balance between efficacy and clinical tolerance is, therefore, a continuing challenge to manufacturers. Moreover, we did not combine the respiratory events with unintentional leaks, which might have further impaired the ability of the devices to maintain sufficient VT.31,32
The mean VT reduction during simulated CA ranged from 16–62%, with only half the devices scoring a residual hypopnea after CA simulation. After OH, a single device scored a residual event based on a aw decrease > 50% for > 10 s. These discrepancies across devices are understandable because no formal recommendations exist for scoring hypopneas during NIV.16,34 In the absence of pulse oximetry and/or arousal detection systems, automatic hypopnea scoring by the device can rely only on flow and pressure variations, resulting in limited sensitivity of hypopnea detection and in variable scoring quality.
Furthermore, NIV is effective only if the upper airway is patent.16 Three devices appropriately increased EPAP in response to OA, although one also increased EPAP during CA. In contrast to OH, OA cannot be terminated by an EPAP increase, as the intraluminal pressure required to open the completely closed upper airway is substantially higher than that required to prevent complete upper-airway closure and is greater than the inspiratory pressure plateau.35,36 Therefore, automatic adjustment seeks to generate an EPAP just above the upper-airway closure pressure once OA is detected then to maintain this level to prevent further occlusion.14,15 For OH, only 2 devices provided an appropriate EPAP response. This finding is of particular concern given that OH accounts for the vast majority of sleep-related events during NIV in patients with chronic hypoventilation.13
An important limitation of this study is that sleep-related respiratory events were simulated for short-term periods. The behavior of the devices may be different over a full night. During the simulated events, low or high velocity of PS changes resulted in undercompensation or overcompensation of VT, respectively. The optimal rate of PS adjustment remains unclear but may be related to the time course of the event responsible for the VT decrease.25 Conceivably, faster adjustment may be required during short-term events, whereas slower adjustment might be more appropriate when compliance decreases due to body position. Furthermore, the BiPAP A40 Pro algorithm is not designed to respond to single events, such as those simulated for our study, but instead continuously assesses airway resistance using the intermittent forced oscillation technique37 and adjusts EPAP to prevent events over the full night. In a randomized controlled study, NIV using this algorithm provided similar benefits to standard PS ventilation in subjects with obesity hypoventilation syndrome, without altering sleep quality or gas exchange.38 This finding suggests that our bench study may have underestimated the effectiveness of this device. Nevertheless, our results underline that clinical trial results have external validity only for the tested device given the possibility of major differences across devices.38,39
Conclusions
Current NIV devices differed substantially in their responses to standardized simulated sleep-related respiratory events. Moreover, event recording in software reports also varied considerably. Clinical guidelines for the management of OA include CPAP titration using automated devices at home.40 The ability to do the same in patients who require NIV is impatiently awaited. However, to meet this expectation, our results suggest a need for further improvements in algorithms embedded in NIV devices.
Supplementary Material
Footnotes
Mr Delorme discloses relationships with Air Liquide Medical Systems, Breas Medical, ResMed, and L3 Medical. Dr Leotard discloses relationships Air Liquide Medical Systems. The remaining authors have disclosed no conflicts of interest.
The devices studied were supplied by ASV Santé, a home care provider involved in the conducting of the current study.
Drs Lofaso and Louis are co-senior authors.
Supplementary material related to this paper is available at http://www.rcjournal.com.
REFERENCES
- 1.Murphy PB, Rehal S, Arbane G, Bourke S, Calverley PMA, Crook AM, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized Clinical Trial. JAMA 2017;317(21):2177-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masa JF, Corral J, Caballero C, Barrot E, Terán-Santos J, Alonso-Álvarez ML, et al. ; on behalf of the Spanish Sleep Network. Noninvasive ventilation in obesity hypoventilation syndrome without severe obstructive sleep apnea. Thorax 2016;71(10):899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonds AK, Muntoni F, Heather S, Fielding S. Impact of nasal ventilation on survival in hypercapnic Duchenne muscular dystrophy. Thorax 1998;53(11):949-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of noninvasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomized controlled trial. Lancet Neurol 2006;5(2):140-147. [DOI] [PubMed] [Google Scholar]
- 5.Patout M, Lhuillier E, Kaltsakas G, Benattia A, Dupuis J, Arbane G, et al. Long-term survival following initiation of home noninvasive ventilation: a European study. Thorax 2020;75(11):965-973. [DOI] [PubMed] [Google Scholar]
- 6.Raphael JC, Chevret S, Chastang C, Bouvet F. Randomized trial of preventive nasal ventilation in Duchenne muscular dystrophy. French Multicentre Cooperative Group on Home Mechanical Ventilation Assistance in Duchenne de Boulogne Muscular Dystrophy. Lancet 1994;343(8913):1600-1604. [DOI] [PubMed] [Google Scholar]
- 7.Cantero C, Adler D, Pasquina P, Uldry C, Egger B, Prella M, et al. Long-term noninvasive ventilation in the Geneva Lake Area: indications, prevalence, and modalities. Chest 2020;158(1):279-291. [DOI] [PubMed] [Google Scholar]
- 8.Kotanen P, Kreivi H-R, Kainu A, Brander P. The prevalence of chronic respiratory failure treated with home mechanical ventilation in Helsinki, Finland. Eur Respir J 2019;54(suppl 63). [Internet][cited 2021 Mar 5] Available from: https://erj.ersjournals.com/content/54/suppl_63/PA2311. [Google Scholar]
- 9.Ergan B, Oczkowski S, Rochwerg B, Carlucci A, Chatwin M, Clini E, et al. European Respiratory Society guidelines on long-term home noninvasive ventilation for management of COPD. Eur Respir J 2019;54(3):1901003. [DOI] [PubMed] [Google Scholar]
- 10.Mokhlesi B, Masa JF, Brozek JL, Gurubhagavatula I, Murphy PB, Piper AJ, et al. Evaluation and management of obesity hypoventilation syndrome. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2019;200(3):e6-e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CH, Finkel RS, Bertini ES, Schroth M, Simonds A, Wong B, et al. ; Participants of the International Conference on SMA Standard of Care. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol 2007;22(8):1027-1049. [DOI] [PubMed] [Google Scholar]
- 12.Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation — a consensus conference report. Chest 1999;116(2):521-534. [DOI] [PubMed] [Google Scholar]
- 13.Aarrestad S, Qvarfort M, Kleiven AL, Tollefsen E, Skjønsberg OH, Janssens J-P. Sleep related respiratory events during noninvasive ventilation of patients with chronic hypoventilation. Respir Med 2017;132:210-216. [DOI] [PubMed] [Google Scholar]
- 14.Selim BJ, Wolfe L, Coleman JM, Dewan NA. Initiation of noninvasive ventilation for sleep-related hypoventilation disorders: advanced modes and devices. Chest 2018;153(1):251-265. [DOI] [PubMed] [Google Scholar]
- 15.Piper AJ. Advances in noninvasive positive airway pressure technology. Respirology 2020;25(4):372-382. [DOI] [PubMed] [Google Scholar]
- 16.Berry RB, Chediak A, Brown LK, Finder J, Gozal D, Iber C, et al. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med 2010;6(5):491-509. [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Bermejo J, Janssens J-P, Rabec C, Perrin C, Lofaso F, Langevin B, et al. ; SomnoNIV group. Framework for patient-ventilator asynchrony during long-term noninvasive ventilation. Thorax 2019;74(7):715-717. [DOI] [PubMed] [Google Scholar]
- 18.Borel J-C, Palot A, Patout M. Technological advances in home noninvasive ventilation monitoring: Reliability of data and effect on patient outcomes. Respirology 2019;24(12):1143-1151. [DOI] [PubMed] [Google Scholar]
- 19.Ambrosino N, Vitacca M, Dreher M, Isetta V, Montserrat JM, Tonia T, et al. ; ERS Tele-Monitoring of Ventilator-Dependent Patients Task Force. Tele-monitoring of ventilator-dependent patients: a European Respiratory Society statement. Eur Respir J 2016;48(3):648-663. [DOI] [PubMed] [Google Scholar]
- 20.Janssens J-P, Borel J-C, Pépin J-L; SomnoNIV Group. Nocturnal monitoring of home noninvasive ventilation: the contribution of simple tools such as pulse oximetry, capnography, built-in ventilator software, and autonomic markers of sleep fragmentation. Thorax 2011;66(5):438-445. [DOI] [PubMed] [Google Scholar]
- 21.Rabec C, Georges M, Kabeya NK, Baudouin N, Massin F, Reybet-Degat O, et al. Evaluating noninvasive ventilation using a monitoring system coupled to a ventilator: a bench-to-bedside study. Eur Respir J 2009;34(4):902-913. [DOI] [PubMed] [Google Scholar]
- 22.Ogna A, Nardi J, Prigent H, Quera Salva M-A, Chaffaut C, Lamothe L, et al. Prognostic value of initial assessment of residual hypoventilation using nocturnal capnography in mechanically ventilated neuromuscular Patients: a 5-year follow-up study. Front Med (Lausanne) 2016;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown LK. Autotitrating CPAP: how shall we judge safety and efficacy of a “black box”? Chest 2006;130(2):312-314. [DOI] [PubMed] [Google Scholar]
- 24.Georges M, Adler D, Contal O, Espa F, Perrig S, Pépin J-L, et al. Reliability of apnea-hypopnea index measured by a home bi-level pressure support ventilator versus a polysomnographic assessment. Respir Care 2015;60(7):1051-1056. [DOI] [PubMed] [Google Scholar]
- 25.Lofaso F, Leroux K, Boussaid G, Prigent H, Louis B. Response of home-use adaptive pressure modes to Simulated Transient Hypoventilation. Respir Care 2020;65(9):1258-1267. [DOI] [PubMed] [Google Scholar]
- 26.Hirose M, Honda J, Sato E, Shinbo T, Kokubo K, Ichiwata T, et al. Bench study of auto-CPAP devices using a collapsible upper-airway model with upstream resistance. Respir Physiol Neurobiol 2008;162(1):48-54. [DOI] [PubMed] [Google Scholar]
- 27.Zhu K, Kharboutly H, Ma J, Bouzit M, Escourrou P. Bench test evaluation of adaptive servoventilation devices for sleep apnea treatment. J Clin Sleep Med JCSM Med 2013;9(9):861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fauroux B, Leroux K, Desmarais G, Isabey D, Clément A, Lofaso F, et al. Performance of ventilators for noninvasive positive-pressure ventilation in children. Eur Respir J 2008;31(6):1300-1307. [DOI] [PubMed] [Google Scholar]
- 29.Oscroft NS, Smith IE. A bench test to confirm the core features of volume-assured noninvasive ventilation. Respirology 2010;15(2):361-364. [DOI] [PubMed] [Google Scholar]
- 30.Contal O, Vignaux L, Combescure C, Pepin J-L, Jolliet P, Janssens J-P. Monitoring of noninvasive ventilation by built-in software of home bi-level ventilators: a bench study. Chest 2012;141(2):469-476. [DOI] [PubMed] [Google Scholar]
- 31.Khirani S, Louis B, Leroux K, Delord V, Fauroux B, Lofaso F. Harms of unintentional leaks during volume targeted pressure support ventilation. Respir Med 2013;107(7):1021-1029. [DOI] [PubMed] [Google Scholar]
- 32.Luján M, Sogo A, Grimau C, Pomares X, Blanch L, Monsó E. Influence of dynamic leaks in volume-targeted pressure support noninvasive ventilation: a bench study. Respir Care 2015;60(2):191-200. [DOI] [PubMed] [Google Scholar]
- 33.Kushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med 2008;4(2):157-171. [PMC free article] [PubMed] [Google Scholar]
- 34.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. ; American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8(5):597-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson SL, Thach BT, Brouillette RT, Abu-Osba YK. Upper-airway patency in the human infant: influence of airway pressure and posture. J Appl Physiol Respir Environ Exerc Physiol 1980;48(3):500-504. [DOI] [PubMed] [Google Scholar]
- 36.Olson LG, Strohl KP. Airway secretions influence upper-airway patency in the rabbit. Am Rev Respir Dis 1988;137(6):1379-1381. [DOI] [PubMed] [Google Scholar]
- 37.Lorino AM, Lofaso F, Duizabo D, Zerah F, Goldenberg F, Ortho M. D, et al. Respiratory resistive impedance as an index of airway obstruction during nasal continuous positive airway pressure titration. Am J Respir Crit Care Med 1998;158(5 Pt 1):1465-1470. [DOI] [PubMed] [Google Scholar]
- 38.Patout M, Gagnadoux F, Rabec C, Trzepizur W, Georges M, Perrin C, et al. AVAPS-AE versus ST mode: a randomized controlled trial in patients with obesity hypoventilation syndrome. Respirology 2020;25(10):1073-1081. [DOI] [PubMed] [Google Scholar]
- 39.Orr JE, Coleman J, Criner GJ, Sundar KM, Tsai SC, Benjafield AV, et al. Automatic EPAP intelligent volume-assured pressure support is effective in patients with chronic respiratory failure: a randomized trial. Respirol Carlton Vic 2019;24(12):1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2019;15(2):335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.