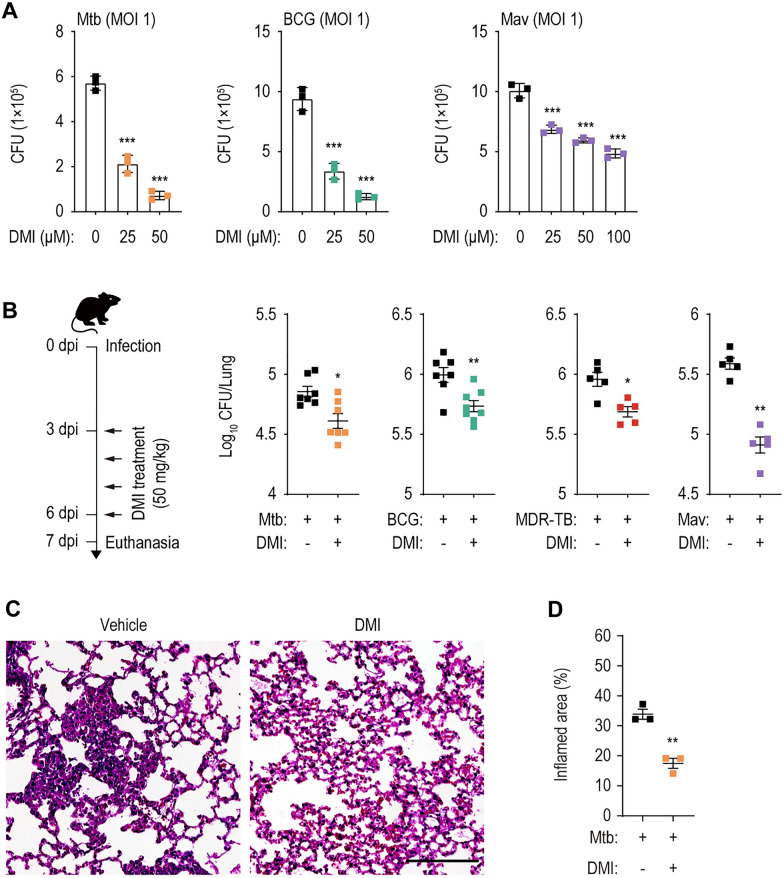
Fig. 1.
DMI-treatment inhibits the both in vitro and in vivo mycobacterial survival. A Intracellular survival of mycobacteria in BMDMs infected with Mtb, BCG, or Mav (MOI 1). BMDMs were infected with Mtb (left panel), BCG (mid panel), or Mav (right panel). After 4 h, cells were washed with pre-warmed DPBS and treated with SC or indicated concentration of DMI. At 3 dpi, cells were lysed and used to a CFU assay to examine the intracellular survival of Mtb, BCG, or Mav. B Mice were intranasally infected with Mtb (5 × 104 CFU, n = 7–8 per group), BCG (1 × 107 CFU, n = 7–8 per group), MDR-Mtb (5 × 103 CFU, n = 5 per group), or Mav (1 × 107 CFU, n = 5 per group), followed by treatment with vehicle or DMI (50 mg/kg) by intraperitoneal (i.p.) injection, and euthanized as depicted schematic diagram of experimental schedule (left panel). The dissected lungs from mice were subjected to analyze the bacterial burden by CFU assay. C, D Mice (n = 3 per group) were infected with Mtb (5 × 104 CFU) followed by treatment with vehicle or DMI (50 mg/kg) by i.p. injection. At 28 dpi, lungs were harvested to determine the inflamed area. Representative histopathological images (C, scale bar = 300 μm) and quantitative analysis for the inflamed area of the lung tissues from mice using H&E (D). Statistical analysis was determined with one-way ANOVA test with Tukey’s multiple comparisons (A) and Mann–Whitney U test (B, D). Data are representative of at least three independent experiments, and error bars denote ± SD (A) or ± SEM (B, D). CFU colony forming unit, DMI dimethyl itaconate, MOI multiplicities of infection, dpi days post infection, MDR-TB MDR-Mtb. *p < 0.05, **p < 0.01, and ***p < 0.001