Figure 3. pH-specific homodimer interfaces regulate ligand-binding.
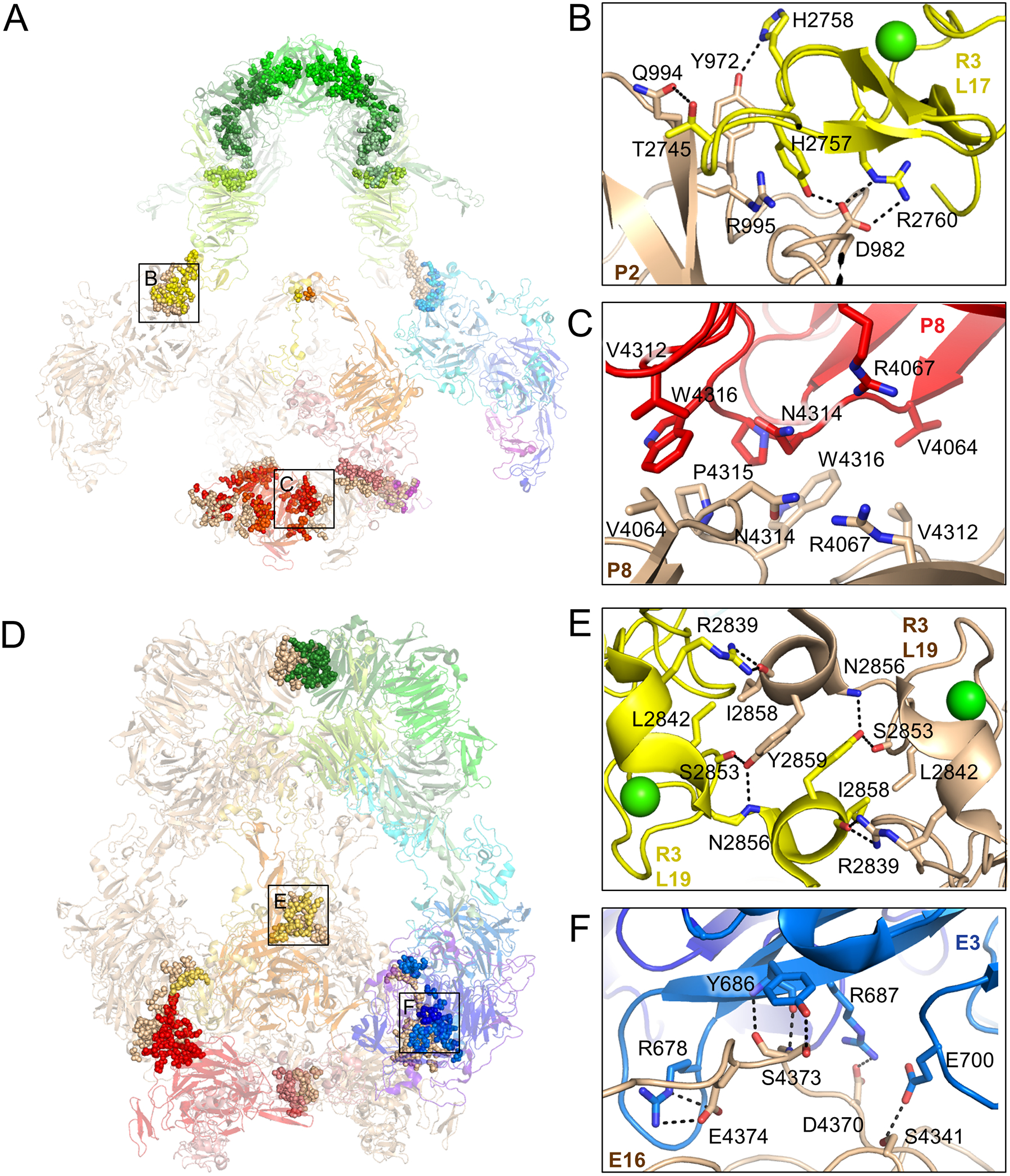
(A) Residues involved in homodimer contacts between protomers at pH 7.5 are rendered as spheres superimposed on a semi-transparent cartoon depiction of the LRP2 structure (left panel). Residue level interactions are shown at right for the corresponding labeled boxed regions. Identities of interacting domains are labeled.
(B) L17 of R3 is stabilized with its ligand-binding face facing solvent by virtue of polar contacts with P2 of the second protomer.
(C) R4067, which functions as an intramolecular ligand at pH 5.2, is buried in a hydrophobic interface between the P8 domains of the two protomers at pH 7.5, preventing it from competing with ligand at ligand-binding repeats.
(D) Depiction and labeling of LRP2 at pH 5.2 according to panel A.
(E) L19 from R3 of the two protomers engage in symmetric contacts that tether the protomers together at the center of the assembly, excluding solvent and making ligand-binding surfaces inaccessible.
(F) E3 of the first protomer packs against E16 of the second protomer in the homodimer, tethering the protomers together near the membrane insertion points.
See also Figure S4
